
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
Networking with AKAPs
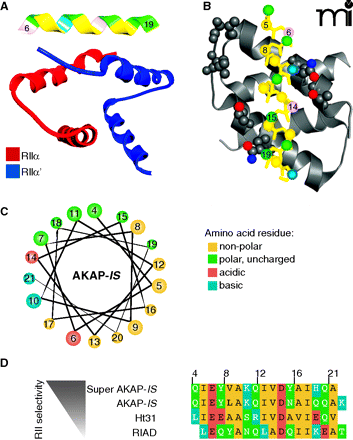
Structural aspects of AKAP interactions with the regulatory subunits of PKA. A. Structure of AKAP-IS in complex with the RII X-type 4 helix bundle. The AKAP-IS helix (top) interacts with the dimerization/docking (D/D) domain of RII through interactions within the core hydrophobic interface (yellow), and polar contacts that form hydrogen bonds with RII. B. Detailed top-view of the protein-protein interactions between AKAP-IS and RII. The residues important for AKAP-RII interactions (hydrophobic and H bonding) are numbered, with corresponding interaction sites in RII highlighted. C. Helical wheel rendering of the AKAP-IS amphipathic helix, showing a face of hydrophobic residues (yellow). D. Sequence alignment of the RII binding domain of AKAP peptides. The pdb accession number 2izx was used in A and B.


