
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
Mechanisms of Pathogenesis in Drug Hepatotoxicity Putting the Stress on Mitochondria
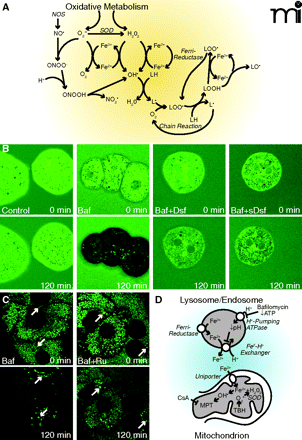
Chelatable iron mobilization and ROS formation. In panel A, oxidative metabolism causes formation of O2•− (i.e., superoxide anion) and H2O2. Superoxide dismutase (SOD) converts some O2•− to H2O2. In the presence of chelatable iron, O2 reduces Fe3+ to Fe2+, and Fe2+ reacts with H2O2 to form hydroxyl radical (OH•). Ferrireductases may also reduce Fe3+ to Fe2+. Hydroxyl radicals react with lipids to form alkyl radicals (L·) to initiate an oxygen-dependent chain reaction generating lipid peroxides (LOOH) and peroxyl radicals (LOO·). Iron also catalyzes a chain reaction generating alkoxyl radicals (LO·) and more LOO·. Nitric oxide synthase (NOS) catalyzes formation of NO•, which reacts with O2•− to form ONOO−. ONOO− decomposes to nitrogen dioxide (NO2·) and OH •. In panel B, the cytosol of mouse hepatocytes was loaded with calcein, and the cells were incubated in culture medium containing calce-in free acid with no further addition (Control), bafilomycin (Baf), bafilomycin plus desferal (Baf+Dsf) or bafilomycin plus starch-desferal (sBaf+Dsf). Note decreased cytosolic calcein fluorescence after bafilomycin, which was blocked by desferal and starch-desferal (see text for details). In panel C, mitochondria and lysosomes of rat hepatocytes were loaded with calcein, and the cells were exposed to bafilomycin (Baf) or bafilomycin in the presence of Ru360 (Baf+Ru), an inhibitor of the mitochondrial calcium uniporter. Note quenching of mitochondrial calcein fluorescence that was suppressed by Ru360. Arrows identify representative lysosomes whose fluorescence increased rather than decreased after bafilomycin (see text for details). In panel D, ferrireductase reduces Fe3+ to Fe2+ in the lysosomal/endosomal compartment. Bafilomycin, as well as ATP deficiency, inhibits the vacuolar proton-pumping ATPase to cause luminal alkalinization and Fe2+ release by apparent Fe2+-H+ exchange. Fe2+ released to the cytosol is taken up by mitochondria via the calcium uniporter as a first hit promoting injury. Mitochondrial oxidative stress by tBH - provides a second hit of O2•− and H2O2 production, and OH• is formed by the Fenton reaction. OH• leads to onset of the CsA-sensitive MPT that causes mitochondrial swelling and uncoupling of oxidative phosphorylation (see text for details).


