Prevention is Better Than Cure: The Case for Clinical Trials of Therapeutic Cancer Vaccines in the Prophylactic Setting
- Andrew Gray1,
- Lisa Yan1 and
- W. Martin Kast1,2,3,4,5
Immunotherapy, a process by which the immune system is made stronger to resist or to fight disease, has long been regarded as a novel strategy for treating cancer. Tumor cells frequently express unique antigenic targets and/or overexpress normal antigens that are usually expressed only at low levels on healthy cells, allowing the immune system to differentiate between cancerous and normal cells. Because of the exquisite specificity of the immune system, cancer cells might possibly be eradicated with minimal collateral damage to surrounding tissues. This exploiting of the immune system’s inherent specificity would provide an enormous advantage over conventional cancer therapies that are characterized by dramatic and overwhelming side-effects that result from their non-specific modes of action. Further, if a therapeutic cancer vaccine is successful in eliciting a strong immune response, very few treatments would likely be needed. Perhaps only an initial priming immunization followed by a few booster shots is all that would be necessary. Combined with relatively cheap off-the-shelf cancer vaccines based on DNA, peptides, proteins and/or viral vectors, this means that immunotherapy may be a comparatively inexpensive treatment regimen for cancer treatment when compared to multiple inpatient chemotherapeutic, radiological, and surgical interventions in conventional cancer therapy.
Recently, immunotherapy was validated as a viable strategy for treating cancer when the Food and Drug Administration (FDA) approved the first true therapeutic cancer vaccine, namely sipuleucel-T for prostate cancer. Sipuleucel-T is an active cellular vaccine that is based on autologous peripheral blood mononucleocytes (PBMC) harvested from an individual patient by leukapheresis and treated ex vivo with a recombinant fusion protein consisting of human prostatic acid phosphatase (hPAP) and granulocyte-macrophage colony stimulating factor (GM-CSF). The PAP protein is a normal human antigen that is frequently overexpressed by prostate cancer cells, whereas GM-CSF acts to stimulate the antigen presenting cells (APC) present within the PBMC isolated from the patient. Upon in vitro stimulation with sipuleucel-T, these APCs become activated and display peptide fragments of PAP bound to major histocompatibility complex (MHC) molecules at their cell surface, thereby allowing the PAP-MHC to interact with and stimulate PAP-specific T cells when transferred back into the patient. To assess the efficacy of sipuleucel-T, 225 men with metastatic hormone-refractory prostate cancer (HRPC) were recruited into two identical randomized, double-blind, placebo controlled phase 3 clinical trials. In total, 147 men were randomly selected for the sipuleucel-T arm and seventy-eight men received the placebo. Three infusions of sipuleucel-T or placebo were administered to each trial subject at intervals of approximately two weeks. The integrated results of these studies demonstrated an improved median overall survival time of 4.3 months in patients that received sipuleucel-T (23.2 months) compared to those that received a placebo (18.9 months) (1). This unprecedented improvement in overall survival was achieved in the absence of severe side-effects, and thus sipuleucel-T became the first therapeutic cancer vaccine to be approved for human use by the FDA.
Despite this extraordinary success, there is still much work to be done. The field is still a long way away from realizing the ambition of immune-mediated tumor eradication. There have been far more failures than successes in clinical trials of cancer vaccines. In decades of research, the standard response of the tumor immunotherapy field to a failed clinical trial has been to attempt to enhance the immunogenicity of the next candidate vaccine. This has involved the identification and targeting of novel tumor-specific antigens; use of different vaccine vectors or types (DNA, protein, peptide, tumor cell lysate, etc.); addition of adjuvants or immunostimulatory cytokines to the vaccine formulation, or a combination thereof. Despite decades of tweaking, the number of cancer immunotherapies that have failed in clinical trials vastly outweighs the single success story to date. Nonetheless, there have been several studies in which a subset of patients that did mount either cellular or humoral immune responses upon administration of the vaccine being evaluated demonstrated longer survival compared to the vaccinated patients that did not mount a response. This implies that at least some of the vaccines that have been in trials are actually capable of eliciting a clinically beneficial immune response, but only in patients who are healthy enough to mount such an immune response (2).
As with all clinical trials of therapeutic cancer vaccines to date, the patient cohort selected for the sipuleucel-T trials consisted of individuals with very advanced disease that had failed all other therapeutic approaches. As has been discussed, these patients are far from being the ideal cohort in which to test immunotherapeutic agents (2). These patients have been subjected to a number of conventional therapies that may be immunosuppressive. Furthermore, advanced tumors fundamentally alter the immune systems of patients, severely limiting their ability to mount an effective anti-tumor immune response upon vaccination. The mechanisms by which tumors may escape immune detection and destruction have been reviewed in depth (3, 4). Briefly, however, tumors are capable of developing or subverting a number of immune tolerance mechanisms which under normal circumstances would be responsible for limiting harmful autoimmune responses. These mechanisms include the recruitment of (and/or conversion of other T cell subtypes to) regulatory T cells and suppressive/tolerogenic dendritic cells (DC), and the production of immunosuppressive cytokines within the tumor microenvironment. In addition, antigen presentation, T-cell activation, and CD8+ T-cell memory function are often compromised within tumors and contribute to immune failure in cancer patients (5). To summarize, advanced tumors develop a microenvironment that suppresses T cell responses (referred to as “tolerizing”), thereby shielding themselves from attack by the patients’ immune system. Therefore, patients with advanced cancer cannot reasonably be expected to be capable of mounting an immune response capable of eradicating a tumor, regardless of the potency of the immunotherapeutic strategy deployed. We and others have hypothesized that inhibitory effects of tumor-mediated immune suppression on the efficacy of cancer immunotherapy can be avoided by vaccinating patients prior to the development of an advanced tumor and its attendant immunosuppressive milieu (2, 6). These considerations have led several groups to attempt the use of therapeutic cancer vaccines in the preventive setting.
The potential of employing cancer vaccines in the preventive setting was elegantly demonstrated very recently by Jaini et al. (7). The authors showed that vaccination against α-lactalbumin, a protein normally only expressed in lactating breast tissue but whose expression is greatly increased in breast cancer cells, yielded outstanding long-term protection against the autochthonous development of breast cancer in a transgenic mouse model of breast cancer. In these transgenic animals, overexpression of the neu receptor protooncogene is driven by the glucocorticoid response element (GRE) found in the long terminal repeat sequence of mouse mammary tumor virus (MMTV) (8). This model recapitulates the overexpression of Her2/neu observed in 25–30% of invasive human breast cancers (9), and is widely used to study the disease. Normally, 50% of MMTV-neu mice would develop spontaneous mammary tumors by 205 days. MMTV-neu mice were vaccinated using either recombinant α-lactalbumin with complete Freund’s adjuvant (CFA) or CFA alone. At ten months of age, all animals were euthanized. None of the α-lactalbumin vaccinated mice developed mammary tumors, in stark contrast to the CFA-only control group which had a 100% incidence rate. In addition, the vaccination strategy was extremely successful in prophylactically inhibiting the growth of implanted 4T1 breast cancer cells. The immune response elicited was characterized a dramatic influx of tumor infiltrating lymphocytes (TIL) into the tumor (predominantly CD4+ T cells but also CD8+ T cells) that produced large amounts of IFN-γ. The CD8+ T cells were capable of directly killing 4T1 cells in vitro. Vaccination against α-lactalbumin was also capable of eliciting less significant therapeutic protection against implanted 4T1 tumors at five and thirteen days post inoculation, but not at twenty-one days post inoculation. This is consistent with the majority of animal studies of cancer vaccines, which frequently show better prophylactic than therapeutic protection. It is exceedingly rare, however, to achieve complete, long-term protection via a prophylactic vaccination as Jaini et al. (7) have done.
The data presented in the prophylactic α-lactalbumin vaccination study are similar to our previous findings in prostate cancer immunotherapy. The effect of vaccination against prostate stem-cell antigen (PSCA) was assessed in an autochthonous animal model of prostate cancer, called the transgenic adenocarcinoma of the mouse prostate (TRAMP) mouse. In these animals, expression of the simian virus 40 (SV40) oncogenic large T antigen is driven by the prostate-specific rat probasin promoter. This results in a line of transgenic mice that develop prostate cancer in a manner that recapitulates the course of disease in humans (10). The overall vaccination strategy used was to prime with DNA encoding mouse PSCA and then to boost with Venezuelan Equine Encephalitis (VEE) virus replicon particles (VRP) encoding the same antigen. This approach yielded outstanding improvements in overall survival, with 90% of vaccinated TRAMP mice still alive and healthy at 340 days, compared to just 10% of control animals (11). Protection against prostate cancer was mediated by tumor-infiltrating CD4+ and CD8+ T cells. As with the Jaini et al. study (7), immunization was administered to animals at eight weeks of age, prior to the development of cancer. At this age, TRAMP mice do have prostate intraepithelial neoplasia (PIN) lesions, but have yet to progress to having full adenocarcinomas or neuroendocrine tumors, which occurs in virtually all TRAMP mice by sixteen weeks of age. Indeed, a follow-up study demonstrated that the efficacy of vaccination in TRAMP mice at sixteen weeks of age was significantly reduced compared to immunization at eight weeks (12). This phenomenon was associated with an increase in intratu-moral regulatory T cells as prostate tumors grew larger.
The animal studies described above demonstrate very aptly the main challenge facing tumor immunotherapy researchers: Excellent cancer vaccines have been developed that work most effectively in the preventive setting, but which can only be tested in clinical trials in gravely ill patients with advanced disease. These individuals are the very same demographic in which the preclinical studies suggest cancer vaccines will be least effective. From this perspective it seems illogical, but there are a number of good reasons why this patient cohort is chosen for clinical trials of cancer vaccines. The first is ethical; the patients recruited to these trials have failed all other therapies. Because they would no longer receive anything other than palliative treatment, it is ethically acceptable to assign some of them to the active arm of the clinical trial and the remainder to a placebo arm. Those who are given the placebo continue to receive the standard of care, whereas in a successful trial, those in the active arm might gain a few extra months as in the sipuleucel-T trials. The second reason this cohort is selected for clinical trails of cancer immunotherapies is cost. By selecting patients with more advanced disease, shorter clinical trials can be conducted using events such as death or time to progression as the primary endpoints of the study. Though this approach has served the oncology research community well for novel chemotherapeutic agents—where the overall health of the patient has less effect on the efficacy of the therapeutic agent—the evidence is mounting that the approach is simply impractical for the assessment of new cancer immunotherapies.
As a result of the ethical and financial considerations discussed above, to date there have been no clinical trials of cancer vaccines targeting autoantigens in the prophylactic setting. There is, however, a precedent for large scale, long-term clinical trials aimed at the prevention of cancer, as opposed to its cure. Several chemoprevention trials have been carried out or are in progress in patients at risk for developing breast cancer or prostate cancer. For example, there is currently a randomized, placebo-controlled, double-blind clinical trial to examine the effect of administering oral anastrozole in women at increased risk of developing breast cancer (NCT00078832). Anastrozole is an aromatase inhibitor that prevents the synthesis of estrogen, thereby limiting the growth of estrogen-dependent breast cancer cells (13). An estimated total of 6000 women between 40–70 years of age will recruited and randomized into two arms, one of which will receive anastrozole daily for five years and one of which will receive a placebo. Subjects in both arms will be followed until the primary outcome measure (the development of histologically confirmed breast cancer, including ductal carcinoman in situ, DCIS) is reached, with an estimated median follow up of five years. The secondary outcome measure is breast cancer mortality, with an estimated median follow-up of ten years. Recruitment for this trial started in 2003 and it is ongoing.
In the case of prostate cancer, there have been several large scale, long-term interventional chemoprevention trials (14). One of these, the Prostate Cancer Prevention Trial (PCPT) was a randomized, placebo-controlled, double-blind study with the aim of determining the effect of daily finasteride treatment on the prevalence of prostate cancer over seven years (15). A total of 18,882 men aged fifty-five years or older were randomized into two arms, one of which received finasteride daily for seven years, and the other of which received a placebo. At the time of recruitment, the subjects had not been diagnosed with prostate cancer and had normal digital rectal examination (DRE) results and serum prostate-specific antigen (PSA) concentrations of 3.0 milligram per liter. A dramatic 24.8% reduction in the prevalence of prostate cancer was observed in the finasteride arm compared to the control arm (p < 0.001). Initially, this success was tempered by an apparent increase of high-grade prostate cancers (Gleason grade 7, 8, 9 or 10) in the finasteride arm, but this was later determined to be incorrect: high-grade prostate cancers were less prevalent in the finasteride-reated subjects (16). Though the PCPT was expensive, costing an estimated $73 million, it reached its primary objective of demonstrating that daily finasteride treatment can reduce the prevalence of prostate cancer in men. Indeed, the PCPT was so successful in reaching its primary objective that it was terminated fifteen months early. In addition, it continues to yield data indicating that finasteride can significantly reduce the risk of high-grade prostatic intraepithelial neoplasia (HGPIN, a precancerous prostate lesion), reduce the risk of radical prostatectomy or radiotherapy, reduce the risk of benign prostate hyperplasia (BPH) related complications and progression, improve the sensitivity and specificity of DRE and PSA screening, and improve the odds of successful diagnosis if prostate cancer is present.
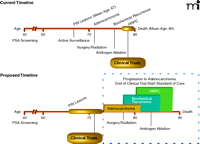
Considering the proven feasibility of long term, large-scale preventive cancer clinical trials and the increasing evidence that cancer vaccines are likely to be far more successful in the prophylactic setting, the tumor immunotherapy field has an opportunity to adopt a new paradigm regarding how it assesses the efficacy of cancer vaccines. Rather than continue to test cancer immunotherapy strategies in end-stage patients in whom there is little chance of a successful outcome, the field can and should begin studies of cancer vaccines in healthy subjects who are at increased risk of cancer. Current and proposed timelines for clinical trials of cancer vaccines targeting prostate cancer are presented in Figure 1. The current timeline involves clinical trials of cancer vaccines in men with metastatic prostate cancer that is resistant to immunotherapy. This is contrasted with the proposed timeline of randomized controlled clinical trials designed to test the hypothesis that therapeutic prostate cancer vaccines used in the prophylactic setting can improve time to progression and mortality in immunocompetent men with precancerous HGPIN lesions.
Schematic representation of prostate cancer immunotherapy trial timelines. The timelines of screening, diagnostic, therapeutic and disease progression events are compared between the current paradigm of therapeutic prostate cancer immunotherapy trials and the proposed structure of studies designed to test the efficacy of prophylactic cancer vaccination. The current timeline shows the structure of cancer vaccine clinical trials performed to date in men with metastatic prostate cancer that is resistant to immunotherapy. It is contrasted with the proposed timeline of randomized, controlled clinical trials of cancer vaccines in the prophylactic setting. These would test the hypothesis that cancer immunotherapies administered prophylactically can increase the time to progression to adenocarcinoma in immunocompetent men who have precancerous PIN lesions. Standard prostate cancer therapies would be administered upon progression to adenocarcinoma; precisely when this occurs will depend on the efficacy of the prophylactic cancer vaccination strategy in delaying time to progression.
There are many challenges facing the adoption of this approach, not least of which is the high cost incurred. They will require very large numbers of subjects, all of whom will need to be followed for many years. These individuals must undergo screening prior to the trials and undergo comprehensive follow-up at regular intervals. In the case of breast cancer, this may involve mammogram plus MRI screening, as is currently recommended for women at increased risk for breast cancer (17). For prostate cancer studies, DRE and PSA screening will be necessary. In both cases, biopsy will be required when indicated to confirm progression to cancer, and in the case of prostate cancer trials an optional end-of-study biopsy should be requested of all subjects regardless of progression. Given that the subjects taking part in the trials are healthy upon enrollment, it will take many years for significant numbers to reach the primary endpoint (time to progression) and even longer to reach the secondary endpoint (mortality). As a result of the large numbers of subjects and the very lengthy and detailed follow-up that is required, large-scale clinical trials of prophylactic cancer vaccines will be expensive. Another challenge is that the only currently FDA approved cancer vaccine, sipuleucel-T, is unsuitable for use in an intervention trial. It is prohibitively expensive for use in a clinical trial involving thousands of subjects. In addition, it is not feasible to require healthy men to undergo leukapheresis procedures for something that may not benefit them. In the case of the cancer chemoprevention trials discussed above, the agents in question—anastrozole and finasteride—were previously approved for use in patients. Anastrozole was approved for use in women with breast cancer after surgery, and finasteride was approved to treat benign prostate hyperplasia and male pattern baldness in men. The chemoprevention trials aimed to establish new indications for these drugs. The fact that there are no suitable approved cancer vaccines to trial for prophylactic use, and that suitable cancer vaccines are not likely to be approved unless they are evaluated in the preventive setting, represents something of a conundrum. It is possible that a suitable therapeutic cancer vaccine will be approved in the relatively near future that can then be evaluated in the preventive setting. A recent phase II clinical trial indicated that PROSTVAC-VF may represent a candidate for this. In this trial, men with metastatic HRPC were primed and boosted with two recombinant viral vectors (Vaccinia and fowl-pox, respectively) encoding PSA and three immune costimulatory molecules (LFA-3, B7.1, and ICAM-1) (18). Though the primary endpoint of the study (progression-free survival) was not met, overall survival did improve, as did median survival time. These results certainly indicate that phase III trials of PROSTVAC-VF are warranted, and it may be the next therapeutic cancer vaccine to be approved. PROSTVAC-VF is an off-the-shelf vaccine consisting of viral vectors that can be used in any individual. Thus, it is a far more feasible candidate for use in a large-scale preventive trial than is sipuleucel-T, which must be tailor-made for each subject. Approval of PROSTVAC-VF, however, is still years away at best; therefore, the results of additional clinical trials of this vaccine in the preventive setting will not be available for decades.
The fundamental question that faces the tumor immunotherapy field now is this: Given the lack of an approved cancer vaccine that might be repurposed for prophylactic use, do the putative risks of testing a currently unapproved cancer vaccine in the preventive setting outweigh the potential benefits of successful prophylactic cancer immunotherapy? The biggest potential risk associated with cancer immunotherapy stems from the fact that self antigens (autoantigens) that are expressed on normal healthy cells in addition to cancer cells are almost invariably targeted. Thus, when administered to immune competent subjects, successful induction of an immune response to those autoantigens by vaccination may result in the autoimmune destruction of any healthy organs that express those autoantigens. The avoidance of harmful autoimmunity must be the top priority of any cancer immunotherapy trial.
A very effective means of avoiding harmful autoimmunity is that used by Jaini et al. in the development of their prophylactic breast cancer vaccine (7). The group targeted α-lactalbumin, an autoantigen that is overexpressed by breast cancer cells but is normally only expressed during lactation. The possibility of autoimmune destruction of healthy organs was dramatically demonstrated in lactating mice that were vaccinated against α-lactalbumin in this study. Inflammation was observed in the mammary tissue of these animals, but did not occur in non-lactating animals. The inflammation was mediated by T cells and, ultimately, led to breast failure as evidenced by the malnourishment and growth retardation of pups. Thus, it is of paramount importance that in any clinical trial involving vaccination of healthy women against α-lactalbumin that the expression of this autoantigen be strictly controlled by avoiding pregnancy. Therefore the group suggested that clinical trials be carried out in women who, at the time of enrollment, have no intention of having children in the future. If pregnancy did occur and the woman experienced autoimmune-mediated breast failure, she would most likely be largely unharmed but would be incapable of breastfeeding the infant, which would have to be raised on artificial formula instead.
An advantage for breast cancer immunotherapy is that the breast is a non-vital organ, thus even if harmful autoimmunity is to occur the patient will likely survive. Similarly, the prostate is a small and non-essential organ. Therefore, it also represents a good candidate for prophylactic cancer immunotherapy trials that pose minimal risk to the test subjects in the event of harmful autoimmune reactions. When our group studied the effect of vaccination against the autoantigen PSCA in TRAMP mice prior to the development of prostate cancer, there was little evidence of harmful autoimmunity. Of the twenty PSCA-vaccinated TRAMP mice, eighteen survived to 340 days, at which point all but two control TRAMP mice had died of prostate cancer. Of the eighteen surviving mice, none produced autoantibodies that could react to other tissues that express PSCA at low levels, including kidney, colon, and normal prostate. Only two of the eighteen produced autoantibodies that could detectably bind to PSCA expressed at the levels found on testis tissue. No evidence of inflammatory responses was found in any of these organs in PSCA-vaccinated TRAMP mice. Similar results were observed when TRAMP mice were vaccinated against another prostate tumor associated autoantigen, six-transmembrane epithelial antigen of the prostate (STEAP) (19). Despite the lack of harmful inflammatory responses, a cellular PSCA-targeted response was elicited by vaccination, which prevented the outgrowth of tumor cells expressing large amounts of PSCA and spared healthy prostate tissue expressing normal amounts of PSCA (12). These data suggest that in healthy men expressing normal levels of prostate tumor-associated antigens, harmful autoimmune reactions induced by prophylactic prostate cancer immunotherapy are unlikely to occur. Care must still be taken, however, as the worst-case scenario is the complete autoimmune destruction of the prostate, whereupon the patient would be rendered infertile. As with studies involving prophylactic breast cancer vaccination, any initial safety trials of preventive prostate cancer immunotherapy that are proposed should be limited to individuals who do not wish to have further children, and who could tolerate the potential loss of prostate function. It should be noted that even if the prostate is eradicated by a harmful autoimmune response, the subject involved would be no worse off than he would be after radical prostatectomy. If prophylactic prostate cancer trials are carried out in men who are at increased risk of progressing to prostate cancer, and thus are more likely to have to undergo radical prostatectomy anyway; the subjects involved might be more likely to accept of the low risk of autoimmune prostate destruction. Indeed, in this eventuality they would probably not suffer many of the significant side-effects associated with radical prostatectomy (i.e., collateral damage during this procedure), owing to the extraordinary specificity of the immune system. Overall, with careful consideration of the antigens targeted and study cohort selected, it is very likely that harmful autoimmunity can be avoided during clinical trials of prophylactic cancer vaccines.
Despite the challenges and potential pitfalls, there are many positive factors that seemingly indicate that conducting clinical trials of cancer vaccines in the preventive setting is a viable approach for future research. Firstly, cancer vaccines undergoing testing must be administered as a short series of prime-and-boost immunizations at the beginning of the study. As a result, there is no reliance on protocol adherence on the part of the subjects over the course of several years, reducing the risk of errors. This was a significant problem in the PCPT, in which the nonadherence rate (calculated as the percentage of days of treatment missed in men who developed cancer or underwent end-of-study biopsy) was 14.7% for the finasteride group and 10.8% for the placebo group. Nonadherence would not be a factor in preventive cancer vaccine trials because any subjects who missed immunizations could be excluded from the study immediately. Secondly, trials could be limited to subjects who have been identified as being at high risk for a particular cancer. This would increase the likelihood of meeting primary (time to progression) and secondary (mortality) outcome measures over the course of a clinical trial, reducing the number of subjects necessary and the overall cost of the trial. For example, immunoprevention trials could be carried out in women identified as at increased risk (20–25% lifetime risk) for breast cancer based on family history as calculated by the Claus and the International Breast Cancer Intervention Study (IBIS) models. Under normal circumstances, these women would undergo regular screening involving mammograms plus MRI scans, as opposed to the standard mammogram-only screening. Women could be recruited to the clinical trial upon being identified as being at heightened risk, whereupon they would be randomized either to a study arm that undergoes prophylactic vaccination and the increased mammogram plus MRI screening or are assigned to a control arm where they receive a placebo and the increased screening protocol. Similarly, men that have been identified by biopsy as having PIN lesions could be recruited into a prophylactic prostate cancer immunotherapy trial. They would be randomly assigned into either into a study arm that receives the prostate cancer vaccine and continues DRE and PSA screening, or would be assigned to a group that receives a placebo and undergoes identical screening. In both cases, the study control groups receive the standard of care for individuals in their risk category for the cancer in question, whilst the other group receives the standard of care plus prophylactic vaccination. Conducting studies of prophylactic cancer immunotherapies in patients at increased risk is also pragmatic from the point of view that they are the most likely targets of any national preventive cancer vaccination program implemented as a result of successful clinical trials. In order to assess the cost-effectiveness of using finasteride as a chemopreventive agent, a Markov decision model analysis based on data from the PCPT was carried out. In a Markov decision model, individuals can only exist in discrete states (e.g., a patient progresses to prostate cancer or does not) and the next state in which they can exist depends entirely on the current state they are currently in (e.g., the patient undergoes radical prostatectomy upon progression to prostate cancer or does not). Using a Markov decision model, the expected percentages of a hypothetical group of patients that undergo each possible disease course and treatment option can be calculated. Given that the average cost of each disease course and treatment pathway is known, the overall cost of treating the population can be determined. In addition, the effects on overall treatment costs can be calculated if variables are altered (such as the administration of a preventive agent) that affect the proportion of patients that undergo particular treatment paths. Using data from the PCPT, such a model was created to assess whether the cost of finasteride chemoprevention was justified by the savings that resulted from lower public health costs of treating men with prostate cancer. This study demonstrated that the hypothetical public health expense of administering finasteride as a chemopreventive agent was dramatically reduced as it was applied to groups with increasing lifetime risk of prostate cancer (20). This finding is to be expected because individuals at high risk are the most likely to benefit from preventive therapies. Thus, patients at increased risk would be the most likely to benefit from immunopreventive treatment strategies and therefore are the best group in whom to perform clinical trials of prophylactic cancer vaccines. A final benefit of conducting long-term, large-scale cancer immunoprevention trials would be the unprecedented number of samples that could be obtained from vaccinated individuals and matched controls. Tumors that progressed and were excised from subjects—and, perhaps, end-of-study biopsies taken regardless of progression (as was done in the PCPT)—could be made available to researchers that would allow studies of unprecedented scale on the effect of vaccination on the tumor microenvironment in humans. This would certainly shed light on the mechanisms of action of cancer immunotherapies in humans.
The remarkable study by Jaini et al. has demonstrated beyond doubt that prophylactic vaccination targeting autoantigens can be a powerful method of preventing cancer development (7). This work has once again highlighted the painful fact that eliciting therapeutic immune responses to established tumors is extraordinarily difficult and of limited benefit even when successful. The public health costs associated with cancer are massive, and there is an urgent need for novel treatments. Most cancer immunotherapy clinical trials to date have involved terminally ill patients, and the results have almost universally been disappointing. Therefore, it is time to acknowledge that active immunotherapies cannot be judged in the same manner as conventional cancer treatments and to begin trials of currently unapproved cancer vaccines in the preventive setting. These trials will be expensive and are potentially risky even with careful planning. They may prove, however, to be the more economical and ethical option, given that of the clinical trials conducted under the current paradigm, only one has yielded an approved therapeutic agent with any possible public health benefit or return on investment. It may be more beneficial to focus our limited resources on a few long term, large-scale immunopreventive clinical trials that could have dramatically beneficial results rather than to spend time, money, and lives unwisely on multiple short-term trials that may yield incremental benefits to public health.
Acknowledgments
This work was supported by National Institutes of Health training grant [ T32 GM 067587] (AG); and the Department of Defense Prostate Cancer Research Program Prostate Cancer Training Award [ DAMD PC073417] (AG).
WMK holds the Walter A. Richter Cancer Research Chair. The authors would like to thank Kimberly Banks, MS, CGC for helpful discussion on assessment of risk for breast cancer based on family history.
- Copyright © 2010
References

Andrew Gray, MRes, is a PhD candidate in the laboratory of W. Martin Kast in the Norris Comprehensive Cancer Center at the University of Southern California. His research interests include investigating the role of tumor-mediated immunosuppression in limiting the efficacy of prostate cancer immunotherapies, and mitigating these effects to enhance the effectiveness of cancer vaccines.

Lisa Yan, BS, is a PhD student in the laboratory of W. Martin Kast in the Norris Comprehensive Cancer Center at the University of Southern California. Her research aims are to determine the functional roles of regulatory T cells in both prostate cancer and HPV-induced cervical cancer

W. Martin Kast, PhD, is a Principal Investigator at the Norris Comprehensive Cancer Center and a Professor of Molecular Microbiology & Immunology, Obstetrics & Gynecology and Urology at the University of Southern California in Los Angeles, CA. His research interests center on therapeutic cancer vaccines, viral and tumor immunology. Address comments to mkast{at}usc.edu; fax 323-442-7760.