
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
Pharmacology of Store-operated Calcium Channels
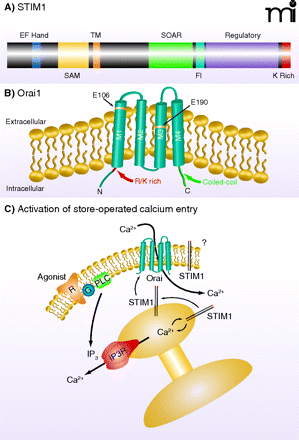
STIM1 and Orai1. A) STIM1 is a single-transmembrane protein localized in the plasma membrane and ER. The N terminus is directed to the lumen of the ER and contains an EF-hand domain that acts as a Ca2+ sensor, followed by a sterile α-motif [(SAM); i.e., a protein interaction domain] and the transmembrane (TM) domain. In the C terminus, within a region of coiled-coil domains, is a STIM–Orai activating region (SOAR), which is involved in activation of Orai channels. Also shown is a region enriched in acidic residues that appears to be involved in fast inactivation (FI) by Ca2+. A regulatory domain contains potential phosphorylation sites that regulate STIM1 function during the cell cycle (61). At the far N terminus is a lysine-rich (K Rich) domain that may contribute to STIM1 localization in near-membrane puncta by interacting with plasma membrane acidic lipids (20). B) Orai channel subunits span the plasma membrane four times, with C and N termini directed to the cytoplasm. Mutations in human Orai1 at positions 106 and 109 alter channel selectivity, providing evidence that Orai proteins are pore-forming units of the CRAC channel. An arginine/lysine rich (R/K Rich) region in the N terminus (99) is involved in coupling Orai to STIM1, as is the coiled-coil C terminus (100). C) Agonism of plasma membrane receptors (R) coupled through a G protein (G) to phospholipase C (PLC) leads to production of IP3, which causes Ca2+ release from the ER through activation of the IP3 receptor (IP3R). The drop in Ca2+ concentration in the ER mobilizes STIM1 to redistribute to near-membrane sites where it activates channels composed of Orai subunits, thereby causing Ca2+ entry. A fraction of cellular STIM1 is also located in the plasma membrane (101) where its function is unclear.


