STAT3 SIGNALING: Anticancer Strategies and Challenges
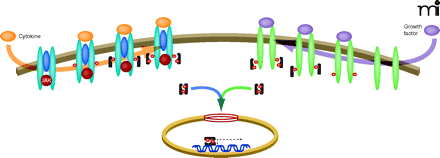
In the canonical STAT3 signaling pathway, activation of cell surface growth factor and cytokine receptors induces specific tyrosine phosphorylation (small red circles) of receptor chains to create docking sites for the recruitment of latent cytoplasmic STAT3 (dark gray geometries), which contain SH-2 domains (shown as indentations) that recognize sites of tyrosine phosphorylation. After the recruitment of STAT3 to activated receptors, STAT3 becomes activated by phosphorylation (at Y705; see text for details). Phosphorylation of STAT3 can be catalyzed by the intrinsic tyrosine kinase activity of the activated growth factor receptor (green elongated ovals) or by the Janus kinase (JAK) that associates with activated cytokine receptors (blue elongated ovals). Phosphorylated STAT3 homodimerizes, via reciprocal intermolecular interactions between SH-2 domains and phosphorylation sites, and dimers translocate into the nucleus. Passage of dimers through the nuclear pore complex (red rings) is facilitated through interactions with importin α5, importin α7, and importin β; nuclear import of nonphosphorylated STAT3 may also occur by importin α3 (not shown). In the nucleus, STAT3 dimers bind to promoter elements of responsive target genes to regulate their transcription. See Tables 1 and 2 for specific activators and targets of STAT3 activity.



