
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
Cytokines in Heart Failure: Potential Interactions with Angiotensin II and Leptin
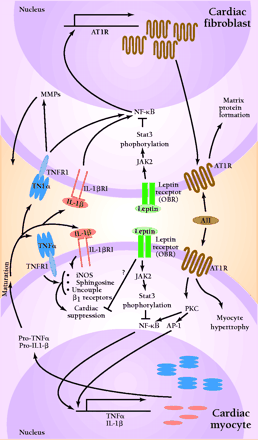
Angiotensin II (AII), acting through angiotensin II type I receptors (AT1R), promotes cardiac myocyte hypertrophy and cardiac fibroblast matrix formation. Activation of AT1R signals the nucleus via protein kinase C (PKC), nuclear factor κB (NF-κB), and activator protein 1-(AP-1)-dependent pathways to increase transcription of tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β). Following protein processing, mature TNF-α and IL-1β are secreted and can act in an autocrine or paracrine fashion to activate their respective receptors. In the cardiac fibroblast, TNFR1 and IL1βR1 activation results in increased activity of matrix metalloproteinases (MMP). Additionally, activation of NF-κB can lead to increased AT1R production. In cardiac myocytes, these cytokines bring about a multifaceted suppression of cardiac function by activating nitric oxide synthase (iNOS), increasing sphingosine production, and uncoupling β1-adrenergic receptors. Leptin, by binding to its receptor (OBR), can activate Janus kinase 2 (JAK2). This results in phosphorylation of signal transducer and activator of transcription (STAT3), which can alter the ability of NF-κB to activate gene transcription. These, as well as other possible leptin-induced processes, have the potential to disrupt AII-cytokine pathways, thus modulating cytokine-induced cardiac suppression.


