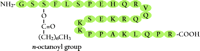Ghrelin and the Regulation of Food Intake and Energy Balance
Abstract
Ghrelin, an endogenous ligand for the growth hormone secretagogue receptor, is synthesized principally in the stomach and is released in response to acute and chronic changes in nutritional state. In addition to having a powerful effect on the secretion of growth hormone, ghrelin stimulates food intake and transduces signals to hypothalamic regulatory nuclei that control energy homeostasis. Thus, it is interesting to note that the stomach may play an important role in not only digestion but also pituitary growth hormone release and central feeding regulation. We summarize recent findings on the integration of ghrelin into neuroendocrine networks that regulate food intake and energy balance.
Introduction
Obesity and related disorders are among the leading causes of illness and mortality in the developed world (1) . To better understand the pathophysiological mechanisms that underlie metabolic disorders, increasing attention has been paid to central regulatory elements in energy homeostasis, including food intake and energy expenditure.
Ghrelin, a 28-amino acid peptide with an n- octanoylation indispensable for its biological activity, was originally discovered in human and rat stomachs as the endogenous ligand for the growth hormone secretagogue (GHS) receptor (GHSR) (2 , 3) . Ghrelin stimulates secretion of GH, food intake, and body weight gain when administrated peripherally or centrally (4 –14) . Ghrelin activates neuropeptide Y (NPY) and agouti-related protein (AGRP)-producing neurons localized in the arcuate nucleus of the hypothalamus (11 , 12 , 15 , 16) , which is one of the brain regions of primary importance in the regulation of feeding. The secretion of ghrelin increases under conditions of negative energy balance, such as starvation, cachexia, and anorexia nervosa, whereas its expression decreases under conditions of positive energy balance, such as feeding, hyperglycemia, and obesity (10 , 17 –24) . Energy homeostasis is accomplished through a highly integrated and redundant neurohumoral system that minimizes the impact on fat mass of short-term fluctuations in energy balance (25 –32) . Crucial elements of this neurohumoral system are hormones such as insulin, leptin, and possibly ghrelin, which serve to integrate the energy and growth needs of the body.
Discovery of Ghrelin
GHSs are a family of small synthetic peptides and non-peptide molecules that stimulate the secretion of GH in several species, including in humans. In 1977, Bowers and colleagues developed the first GHS peptides derived from Met-enkephalin, which stimulated in vitro the release of GH from pituitary cells, although the potency of these peptides was rather weak (33 , 34) . Further development led to the production of several new peptides with increased potency, including GH-releasing peptide-6 (GHRP-6, His–d-Trp–Ala–Trp–d-Phe–Lys-NH2 ) and hexarelin, both of which are active in vitro and in vivo (35 , 36) . Based on the structure of GHRP-6, peptidomimetics of GHS such as MK-0677 (a spiroindoline with marked bioavailability and long-lasting effects after oral administration) were developed by Merck (35) .
The primary action of the GHSs is the stimulation of GH release from the somatotroph acting through a mechanism distinct from that of GH-releasing hormone (GHRH). GHRP-6 activates the phospholipase C (PLC) pathway, resulting in an increase the intracellular Ca2+ through inositol 1,4,5-trisphosphate-(IP3 )-mediated signal transduction, a pathway distinct from the adenosine-3’,5’-monophosphate-(cAMP)-dependent protein kinase (protein kinase A, PKA) pathway utilized by the GHRH receptor (37 –39) . In 1996, the Merck group identified and cloned the GHSR by utilizing expression cloning and exploiting their compound MK-0677. Changes in intracellular calcium concentrations were indicative of a positive response (40 –42) .
Kojima et al. used GHSR-expressing Chinese hamster ovary (CHO) cells to monitor changes in intracellular calcium concentrations that were induced by rat tissue extracts; the highest levels of GHSR activation were observed in response to stomach extracts (2) . The purified ligand, named ghrelin (“ghre” is the Proto-Indo-European root of the word “grow”), has a unique post-translational modification: the hydroxyl group of the third residue (Ser3 ) is esterified by octanoic acid and is essential for ghrelin’s biological activities (Figure 1⇓). Human ghrelin is identical to rat ghrelin apart from two amino acids; however, amphibian (bullfrog) ghrelins undergo acylation at Thr3 (43) . Thus, the acyl modification of the hydroxyl group on the third residue represents an invariant and essential covalent change for the activation of ghrelins across multiple species.
The structure of human ghrelin. Ghrelin is a 28 amino-acid peptide, in which Ser3 is modified by n- octanoic acid. This modification is essential for the activity of ghrelin.
The second endogenous ligand for GHSR was also isolated from the rat stomach (44) . The ligand des -Gln14 -ghrelin, a 27-amino acid peptide with an n- octanoyl modification at Ser3 , is identical to ghrelin except for deletion of one glutamine, and is produced through alternative splicing of the rat ghrelin gene. Des -Gln14 -ghrelin has the same potency as ghrelin for inducing increases in intracellular Ca2+ concentrations in GHSR-expressing cells, and for increasing plasma GH concentrations in rats. On the other hand, the unmodified, des -n- octanoyl form of ghrelin (des -acyl ghrelin), has no effect on the elevation of intracellular Ca2+ and GH secretion. In structure–activity analysis, the octanoic acid is not the only modification group of the Ser3 side chain that can sustain the activity of ghrelin; some other fatty acid modifications can also maintain activity (45) .
Tissue Distribution of Ghrelin
Ghrelin is predominantly produced by the stomach, whereas substantially lower amounts are derived from the bowel, pancreas, pituitary, kidney, and placenta (2 , 18 , 46 –50) . The ghrelin content of the central nervous system is low (46) ; however, by means of immunohistochemical analysis, ghrelin-producing neurons can be demonstrated in a very limited region in the arcuate nucleus of the hypothalamus (2) . These other sources of ghrelin act to increase ghrelin secretion in a compensatory manner or might participate in a paracrine manner. Rat ghrelin is present from the stomach to the colon, with the greatest amount in the gastric fundus (47) . Ghrelin-producing cells, which are not histamine-secreting enterochromaffin-like cells, somatostatin-secreting D cells, or serotonin-secreting enterochromaffin cells, account for about twenty percent of the endocrine cell population in rat and human oxyntic glands (47) . Rat gastric ghrelin is present in a distinct cell type, X/A-like cells, whose hormonal production and physiological functions have not previously been clarified. The X/A-like cells, now designated ghrelin-producing cells, are not in continuity with the stomach lumen but rather are closely associated with the capillary network of the lamina propria, supporting an endocrine role. The amount of ghrelin is very low in the fetal stomach and increases in an age-dependent manner (51) . The concentrations of plasma ghrelin also increase postnatally in parallel with the amount of ghrelin produced by the stomach (52) .
Ghrelin Secretion
The concentrations of ghrelin peptide in the blood and ghrelin mRNA in the stomach increase during fasting and decrease during refeeding in rodents (10 , 20) . Interestingly, sugar intake, but not stomach expansion, is thought to diminish the amount of circulating ghrelin. In contrast, the ghrelin peptide contents in the stomach significantly decrease after fasting and increase after re-feeding. This inverse pattern of ghrelin levels in the stomach tissue and plasma may result from increased secretion of ghrelin from the stomach in response to fasting and subsequent decreased secretion upon resumption of feeding. Two major forms of ghrelin peptide, active n- octanoyl ghrelin and inactive des -acyl ghrelin, were found in the blood and the stomach, with the ratio of n- octanoyl ghrelin to des -acyl ghrelin slightly increased after fasting (53) , suggesting that the modification by n- octanoic acid is an important step for the regulation of the activity of ghrelin. In humans, circulating ghrelin levels are decreased in chronic (obesity) (21) and acute (recent caloric intake) (18 , 22 –24) states of positive energy-balance, whereas ghrelin levels are increased by fasting (10 , 23) and in cachectic or anorexic patients (18 , 19 , 54) .
Ghrelin and GH Secretion
Because GHS is an agonist of the GHSR, it was reasonable to expect that ghrelin possessed GH-releasing activity. Indeed, ghrelin stimulates GH release from primary cultured pituitary cells directly in a dose-dependent manner (2) . In rats, large secretions of GH were observed following intravenous (IV), intraperitoneal, subcutaneous and intracerebroventricular (ICV) injection of ghrelin (2 , 8 , 9 , 12) , indicating that ghrelin is (directly or indirectly) a GH-releasing peptide. Intravenous ghrelin administration in healthy humans potently stimulates GH release (5) , whereas adrenocorticotrophic hormone, cortisol and prolactin levels are also elevated slightly after ghrelin injection (5 , 55 , 56) . The coadministration of ghrelin and GHRH synergistically affect GH secretion (57) .
The GH neuroendocrine axis is sensitive to changes in nutritional status (58) . Plasma ghrelin levels are elevated during fasting in normal subjects, in patients with anorexia nervosa, and those patients with cardiac cachexia (59) whose disease is associated with increases in GH production. Plasma ghrelin levels are negatively correlated with BMI (18 , 21) . Moreover, GH secretion, deeply impaired in human obesity, increases after weight loss and fasting (60 –64) ; however, no correlation between GH and ghrelin secretary patterns has been observed in rats (65) . Prior administration of GHRH antagonists blocks nearly all GHRP-6–dependent GH secretion in humans (66) . In patients with hypothalamo-pituitary disconnection—a transection of the pituitary stalk—the release of GH was severely impaired after treatment with GHRP-6 alone or GHRP-6 plus GHRH, suggesting that the main actions of GHRP-6 (and its synergistic effect with GHRH) are exerted at the hypothalamus (67) . GHRH and somatostatin can also regulate the pulsed release of GH (68) . We have found that ghrelin might augment GH secretion from the pituitary through the afferent vagus nerve system (69) . Peptidyl GHSs and hexarelin induce GH secretion that is associated with GHRH release into hypophysial portal blood in sheep (70) . Thus, we speculate that ghrelin might be the driving force behind enhanced GH secretion, perhaps acting by modulating GHRH levels.
Ghrelin Stimulates Food Intake
GHS causes a short-lived increase in food intake when administered either systemically or ICV (71 –73) . The orexigenic or appetite-stimulating effect of GHS is not altered by pretreatment with a GHRH antagonist at a dose that completely blocks the feeding response to ICV-administered GHRH, indicating that GHSR mediates the effect on appetite (72) . Both the peripheral and central administration of ghrelin also stimulated food intake in freely feeding mice and rats, and in GH-deficient dwarf rats (9 –12 , 15) . ICV administration of ghrelin above a minimally active dose (∼10 pmol) to free-feeding rats increased food intake in a dose-dependent manner (11) . A GHSR antagonist suppressed ghrelin-induced feeding and furthermore, the administration of ghrelin-specific antibodies suppressed starvation-induced feeding in a dose-dependent manner, suggesting that ghrelin is a powerful, endogenous orexigenic peptide. In humans, IV bolus injection (4 , 74) or infusion (14) of ghrelin induces hunger.
Unlike ghrelin, most other hypothalamic peptides—for example, neuropeptide Y (NPY), agouti-related peptide (AGRP), orexins, melanin-concentrating hormone (MCH), and galanin—that stimulate feeding when administered centrally are ineffective when administered into the periphery (Table 1⇓). Ghrelin is the first identified circulating hormone that promotes feeding following systemic administration. In humans, plasma ghrelin levels rise sharply before and fall shortly after every meal (23 , 24) . These findings indicate that ghrelin may serve as an indicator of short-term energy balance and might be a candidate for a meal-initiation signal.
Neuropeptides Implicated in the Control of Energy Homeostasis in the Central Nervous System
Ghrelin and Energy Balance
Chronic ICV administration of ghrelin strongly stimulates feeding in rats and increases body weight gain (11) . Daily subcutaneous administration of ghrelin in mice induces a progressive increase in body weight, with a significant gain in fat mass but no change in lean body mass. This could result from a chronic decrease of fat oxidation as indicated by an increased respiratory quotient (10) . Because ghrelin can induce adiposity that is sustained during ghrelin treatment, ghrelin might participate in the long-term regulation of body mass. In humans, studies suggest that weight-loss increases circulating ghrelin levels (75 , 76) .
GH is also known to be an important anabolic agent, and has been used to counteract muscle wasting associated with surgical stress, sepsis, glucocorticoid administration, and AIDS (77) . Ghrelin secretion counteracts further decreases in energy storage and prevents starvation or cachexia. Therefore, evaluation of the role of ghrelin in the pathogenesis and treatment of such cachectic conditions is warranted (78) . Ghrelin and GH might serve as anabolic signaling molecules during energy depletion.
Ghrelin and the Central Regulation of Feeding
Ghrelin is the first identified hormone that acts as a starvation-signaling molecule from the stomach and stimulates feeding after peripheral administration. After ghrelin is administered to the CNS, neurons expressing the immediate-early transcription factor c-Fos are observed primarily in regions implicated in the regulation of feeding behavior, including the paraventricular nucleus, the arcuate nucleus (ARC), and the dorsomedial and ventromedial hypothalamic nuclei (11) , suggesting that ghrelin might contribute to central control of energy homeostasis such as body temperature and energy expenditure. This distribution coincides with that of GHSR (79) . GHSR mRNA is expressed in 94% of the neurons in the ARC that express NPY, in 8% of cells that express pro-opiomelanocortin (POMC), in 30% of those that express somatostatin, and in 20–25% of those that express GHRH mRNA (80) .
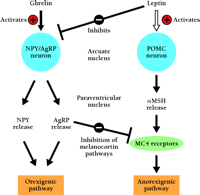
The ARC is an important site for translating input from diverse hormonal signals into behavioral and metabolic responses that powerfully influence energy balance (28 , 81) . NPY and AGRP—orexigenic molecules—are expressed in the same neurons in the medial ARC (82 , 83) , whereas POMC and the cocaine- and amphetamine-regulated transcript (CART)—anorexigenic molecules—are expressed in the lateral ARC (84) . AGRP antagonizes the actions of α -melanocyte-stimulating hormone (α -MSH) at the melanocortin 4 receptor (MC4-R), and is therefore orexigenic (85 , 86) (Figure 2⇓). ICV administration of ghrelin leads to increases in the expression of both NPY and AGRP mRNAs, and pretreatment with NPY-specific or AGRP-specific antibodies, or with a antagonists to NPY or AGRP receptors significantly inhibits ghrelin-induced feeding (11 , 12) . Because ghrelin administration did not alter the expression of POMC mRNA, these results indicate that ghrelin-dependent orexigenesis is mediated by the output of the ARC NPY–AGRP neurons. The ARC is a crucial target of leptin, an anorexia-mediating molecule produced from adipose tissue (28 , 29) . Most NPY–AGRP–producing, or POMC–CART–producing neurons also express leptin receptors, and both types of neurons are regulated by leptin, albeit in an opposing manner (87 , 88) . Leptin inhibits ghrelin-induced feeding, and ghrelin substantially reverses the anorexic effect of leptin, indicating that ghrelin may antagonize leptin action in regulating the NPY–AGRP system (Figure 2⇓).
A simplified model of the feeding-regulatory signaling of ghrelin and leptin. Leptin stimulates the POMC anorexigenic pathway and inhibits the NPY–AGRP orexigenic pathway, resulting in reduced food intake. The effect of ghrelin in the hypothalamus is opposite to that of leptin. The orexigenic effect of ghrelin is mediated by activating on the output of the NPY–AGRP neurons. Fasting increases ghrelin and decreases leptin production, leading to the activation of the orexigenic pathway. This response might be important for the adaptation to fasting.
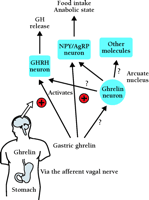
The orexigenic effect of ghrelin might be mediated by the afferent activity of the vagus nerve (12) . Cholecystokinin (CCK) is known to inhibit food intake and gastric emptying by activating gastric vagal afferent nerves (89 , 90) . IV-administered ghrelin significantly decreases the activity of the afferent gastric vagal nerve at doses below those effective for CCK-mediated activity, suggesting that ghrelin’s effects are more powerful than those of CCK (12) . Centrally or peripherally administrated ghrelin can stimulate gastric contraction and stomach emptying in rodents (12 , 91) , and gastric emptying is significantly correlated with high plasma ghrelin levels during fasting (22) . The effect of ghrelin on gastric contraction is probably mediated by the vagal cholinergic pathway. The effects of ghrelin on feeding, gastric emptying and vagal nerve activity oppose those effects of other gastrointestinal peptides that are considered components of the peripheral satiety system (92 –94) . Basic questions such as whether the regulation of GH secretion and food intake are due to stomach and arcuate nuclei-derived ghrelin need to be addressed. Intravenously administered ghrelin induces Fos expression only in the ARC (95) , whereas centrally administrated ghrelin induces Fos expression in various brain regions (11) . These findings imply that a neural pathway mediated by peripherally administered ghrelin differ from a pathway mediated by centrally administered ghrelin (Figure 3⇓).
The orexigenic and somatotrophic effect of peripheral ghrelin mediated by the afferent vagal nerve . Circulating ghrelin acts on the stimulation of feeding and secretion of GH through the gastric vagal afferent nerves, and through the surgical deafferentation of them blocked GH secretion and food intake induced by peripheral administration of ghrelin. Although ghrelin is also produced by hypothalamic cells, it is unclear whether this source of ghrelin acts in a similar fashion to ghrelin produced by the stomach.
The isolation of ghrelin can be considered a landmark in the GH field, allowing for new insights into understanding the regulation of the GH system, food intake, and body fat composition. Ghrelin may prove to be an effective probe for elucidating the normal and pathological regulation of GH release in humans, and should be explored in the clinical setting as a potential diagnostic and therapeutic tool.
Acknowledgments
Research in the authors’ laboratory is supported by grants from the Ministry of Education, Science, Sports and Culture of Japan, Ministry of Health, Labor and Welfare of Japan, and the Promotion of Fundamental Studies in Health Science from the Organization for Pharmaceutical Safety and Research (OPSR) of Japan.
- © American Society for Pharmacology and Experimental Theraputics 2002
References
Hiroshi Hosoda, MD, PhD, (right) is an Instructor of Translational Research Center at Kyoto University Hospital. Masayasu Kojima, MD, PhD, (left) is a Professor of Institute of Life Science at Kurume University. Kenji Kangawa, PhD, is a Professor and Chair of Translational Research Center at Kyoto University Hospital and Department of Biochemistry at National Cardiovascular Center Research Institute. Address correspondence to KK. E-mail kangawa{at}ri.ncvc.go.jp; fax +81-6-6835-5402.