
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
The gβγ Dimer as a Novel Source of Selectivity in G-Protein Signaling: GGL-ing AT CONVENTION
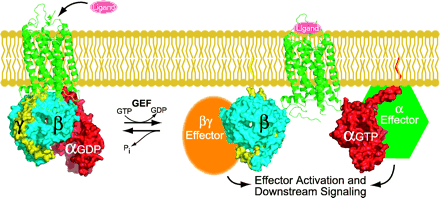
Standard model of heterotrimeric G-protein signaling. Heterotrimeric guanine nucleotide–binding proteins exist in the basal state as a GDP-bound Gα subunit (red) bound to a Gβγ dimer (blue and yellow). Ligand-bound receptor (green), serving as a guanine nucleotide exchange factor (GEF) for the Gα subunit, catalyzes the release of GDP and the binding of GTP. GTP-bound Gα separates from the Gβγ dimer and thus allows Gα and Gβγ to regulate their respective effectors. The cycle is completed upon hydrolysis of GTP to GDP, resulting in reassociation of the GDP-bound Gα with Gβγ. The Gα subunit has an intrinsic guanine nucleotide triphosphatase (GTPase) activity, which can be stimulated by regulators of G protein signaling (RGS proteins) and, in some cases, the Gα effector.


