
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
The gβγ Dimer as a Novel Source of Selectivity in G-Protein Signaling: GGL-ing AT CONVENTION
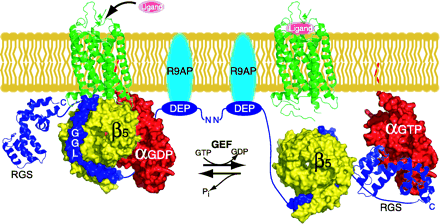
Figure 5.
A model depicting a possible Gα–GDP–Gβ5–R7 heterotrimer coupled to a GPCR. GDP-bound Gα would undergo ligand-induced nucleotide exchange when coupled with a Gβ5–R7 dimer. The Gβ5–R7 complex would be tethered to the membrane via binding of R9AP (or an R9AP-like molecule) to the DEP domain of the RGS protein. Upon activation, the RGS domain of the Gβ5–R7 complex would be conveniently localized to accelerate the hydrolysis of GTP to GDP.


