
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
Purine Receptors: GPCR Structure and Agonist Design
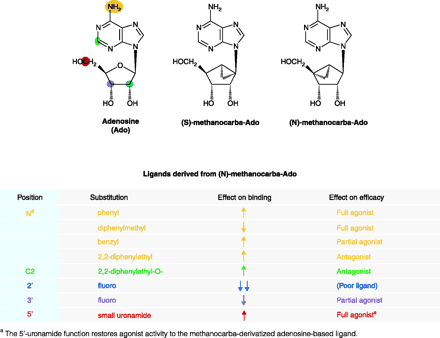
Differential effects of ligand derivatized on binding affinity and functional efficacy. The substitutions of adenosine as tabulated below the structures are offered as general examples of effects that can be observed from ligand derivitization. (Yellow indicates the N6 -position; green, C2-position; blue, 2′-position; purple, 3′-position; red, 5′-position.) The ribose moiety of the adenosine (Ado) ligand becomes effectively fixed in either the (N) or (S) conformation upon replacement with the methanocarba ring system as shown. (For a conformational representation of the (N)-fixed ring system, see the chemical structure shown in Figure 4.) Because the A3 AR prefers the (N)-conformer >100-fold relative to the (S)-conformer, the (N)-methanocarba derivative of adenosine has been used to synthesize novel A3 AR ligands of high affinity (some of which are shown in Figure 3). The (N)-methanocarba modification alone improves ligand binding to the A3 AR and reduces functional efficacy of the receptor, resulting in a partial agonist. [See (30, 35, 39)].


