
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
Purine Receptors: GPCR Structure and Agonist Design
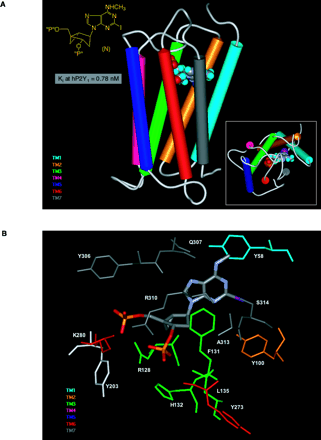
Docking of the high-affinity antagonist MRS2500 at the human P2Y1receptor. A. The basis for the conformational constraint is the methanocarba ring system, in which cyclopentane and cyclopropane rings are fused. The placement of this ring fusion at the 4′ carbon assures that a North (N) conformation is maintained. The large enhancement of affinity produced by this specific conformation within the P2Y receptor family is limited to the P2Y1 receptor (with the possible exception of P2Y14 , which is not yet characterized) (27). “P” represents a monophosphate group. The inset shows the docking as viewed from the extra-cellular side. B. A more detailed image indicates three basic residues, Arg 128 (3.29), Lys 280 (6.55), and Arg 310 (7.39), that putatively coordinate the phosphate moieties (49). The importance of these three amino acid residues in recognition of nucleotides is supported by mutagenesis studies (32). The residues Arg128 and Gln 307 occur roughly one helical turn below the exofacial side of TMs 3 and 7, respectively. [See also (32, 49).]


