
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
Coregulators in Nuclear Estrogen Receptor Action
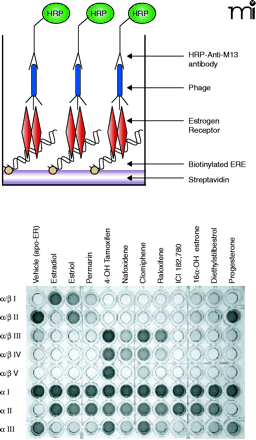
Different ligands induce distinct structural alterations in ERα. A. A drawing indicating the various components of an enzyme-linked immunosorbent assay (ELISA) used to identify distinct changes in ER structure as mediated by binding to peptides displayed on the surface of phage. B. Typical results from a ER-phage ELISA utilizing ERE-containing oligonucleotides. Purified ERα was added in saturating concentrations and then incubated with differing ligands, including the agonist 17β-estradiol and its metabolites estriol and 16α-OH estrone; the ER pure antagonist ICI 182,780; the selective estrogen receptor modulators (SERMs) tamoxifen, nafoxidene, raloxifene; the pharmaceutical agents premarin, clomiphene, and diethylstilbestrol; and solvent and progesterone as negative controls (functionally equivalent to apo-ERα). Individual phage clones, each expressing specific peptides (α/β I, α/βII, α/β III, α/β IV, α/β V, α I, α II, α III) were incubated with the (oligo)DNA-receptor-ligand complexes, unbound phage were washed away and bound phage were detected with an M13 antibody conjugated to HRP. Assays were developed with 2,2-azinobis(3-ethylbenzothiazoline)-6 sulfonic acid and hydrogen peroxide for 10 min and stopped by the addition of 1% SDS. The visual results of the colorimetric assay are shown [Figure adapted from (63)].


