
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
Lights on for Low Oxygen: A Noninvasive Mouse Model Useful for Sensing Oxygen Deficiency
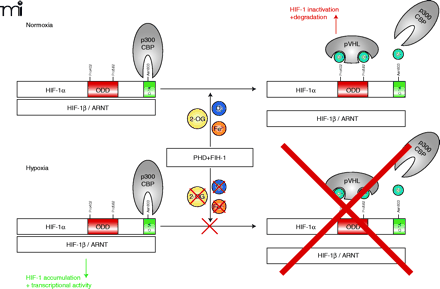
Oxygen concentration-dependent regulation of HIF-1α. Under normoxic conditions (and in the presence of 2-OG and Fe2+)HIF-1α is hydroxylated at two proline residues by specific prolyl-4-hydroxylases (PHDs) which allows the E3 ubiquitin ligase pVHL to bind to HIF-1α and mark it for proteasomal degradation. In addition, HIF-1α is regulated by the aspargine hydroxylase factor inhibiting HIF-1 (FIH-1) in the C-terminal transactivation domain (C-TAD), which inhibits p300/CBP binding to HIF-1α (20). If oxygen concentrations decrease, the PHDs and FIH-1 are inactivated because of the limited oxygen supply. This inactivation allows HIF-1α to accumulate, form the active transcription factor HIF-1 with the α-subunit, recruit transcriptional cofactors, and initiate the transcription of responsive genes. HIF-1, hypoxia-inducible factor; pVHL, von Hippel Lindau protein; ARNT, aryl hydrocarbon receptor nuclear translocator; ODD, oxygen-dependent degradation domain; 2-OG, 2-oxoglutarate.


