
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
Advanced Drug Delivery Systems That Target The Vascular Endothelium
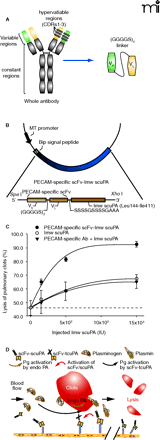
Vascular immunotargeting of recombinant fusion protein combining a single chain variable fragment of anti-PECAM and urokinase (scFv/uPA). A. A schematic representation of different antibody formats. Left side of panel shows generic structure of a whole immunoglobulin G (IgG) molecule, comprising two heavy and light chains linked by disulfide bonds. Hypervariable regions (CDRs) forming antigen binding sites are indicated. Right side of panel shows structure of a single-chain Fv (scFv) in which variable domains of light chain and heavy chain are covalently linked by a flexible inter-chain linker. As depicted, the size of scFv (30 kD) is six-fold smaller than whole IgG molecule (180 kD). B. A schematic diagram describing the cloning strategy for the fusion construct scFv/uPA. Variable domains of antibody heavy chain and light chain were linked and then fused to the N terminus of lmw-scuPA by a (Ser4Gly)2Ala3 linker. C. Pulmonary thrombolysis in mice by PECAM-specific scFv-uPA. The graph shows the dose-response curve of dissolution of pulmonary thrombi by bolus injection of equal doses of PECAM-specific scFv/uPA or non-targeted uPA in mice. Thrombolytic potency was expressed as percent of fibrinolysis vs dose administered. Dash line indicates spontaneous lysis. D. A simplified diagram of a proposed strategy for throm-boprophylaxis using vascular immunotargeting of genetically engineered scFv/uPA. scFv/uPA circulates in a form of a prodrug, binds to PECAM-1, and remains anchored on the luminal surface of endothelium for at least several hours. In situ thrombosis or embolism induces initial local conversion of plasminogen (Pg) into plasmin (Pn) by endogenous plasminogen activators (Endo-PA). Plasmin (and perhaps other enzymes) formed in the vicinity of the clot converts the endothelium-bound scFv/uPA into enzymatically active tcuPA, which in turn amplifies local formation of plasmin, reinforcing local thrombolysis, and preventing clot extension and reocclusion.


