Animal Models and Brain Circuits in Drug Addiction
- Address correspondence to PK. E-mail kalivasp{at}musc.edu; fax 843-792-4423.
Abstract
Animal models in the field of addiction are considered to be among the best available models of neuropsychiatric disease. These models have undergone a number of refinements that allow deeper understanding of the circuitry involved in initiating drug seeking and relapse. Notably, the demonstrable involvement of classic corticostriatal habit circuitry and the engagement of prefrontal cortical circuits in extinction training may have relevance to the therapeutic modulation of habit circuitry and drug addiction in humans.
Introduction
The field of addiction biology, research has been fortunate to have access to what may be the most well developed and valid animal models of a neuropsychiatric disorder (1). Put simply, laboratory animals in controlled settings self-administer the same addictive drugs that humans choose to self-administer for recreational purposes. That these drugs are able to reinforce drug-seeking behavior in all mammalian species examined to date strongly supports the use of self-administration models in studying the acute rewarding properties of drugs, the development of habitual drug-seeking behaviors, and ultimately, addiction. Accordingly, the last thirty years have seen an abundance of publications and scientific careers built around studying animals that have been trained to self-administer addictive drugs.
In spite of the availability of a seemingly excellent animal model, drug addiction remains among the most costly neuropsychiatric disorders in terms of personal tragedy and overall societal economic burden. How is it possible that we have such a well developed animal model and so little success in treating addiction? Is the model fundamentally flawed? Are we using the model inappropriately? Are we expecting too much, too soon? Such questions are best answered in recognition of the complexities of physiologically adaptive behavioral responses. The task of developing an understanding of the pharmacologically induced changes that underlie neuronal signaling and circuit organization in the context of behavioral neuropathologies is quite daunting. Nonetheless, available animal models that produce motivationally driven behavior that recapitulates human addiction—and that the experimenter can manipulate—have already given us important glimpses into the effects of environmental perception, learned associations, and genetic dispositions on normal behavior. Indeed, animal models of addiction offer important opportunities to probe the physiological interfaces between perception, motivation, and behavior.
A number of reviews and perspectives over the last decade have described addiction as a pathology of learning and memory (2). This view can be distilled into two consensus neurobehavioral phases. The first of these holds that the development of addiction is a form of over-learning that is promoted by pharmacological agents that elicit the release of large, non-physiological amounts of dopamine into cortical, allocortical, and striatal brain regions (3). In the second phase, the expression of drug-seeking behavior (i.e., relapse) results from activation of the over-learned drug associations that drive the behavior in a manner that is difficult to regulate. Work over the last decade reveals that the expression of drug seeking is likely linked to altered cortical and allocortical glutamatergic drive into the striatum (4).
The first phase (i.e., development) in human addiction refers to the transition from social, controlled use of addictive drugs into habitual, compulsive patterns of use that disrupt socially adaptive goals (e.g., friendship, work, raising children). The second phase (i.e., expression) in human addiction refers to the process of activating the motivational drive to obtain drug and subsequently relapsing to drug taking. Consistent with addiction being a pathology of learning and retrieval mechanisms, the division of addiction into development and expression was derived from earlier literature describing the two phases in terms of neuroplasticity thought to underlie learning, such as long-term potentiation or electrical kindling of the amygdala (5). Indeed, the classic concepts of behavioral habituation and sensitization are often divided into development and expression components, which were in turn adapted to the locomotor sensitization produced by repeated injection of many addictive drugs (6).
Conceptualizing addiction into development and expression phases has been carried over to the self-administration animal model (7, 8). Thus, the acquisition and maintenance of drug self-administration collectively correspond to the development of addiction, and the reinstatement of drug seeking after a period without drug models the expression of addiction. In this short review, we will focus on the reinstatement model of the expression of addictive behavior and the underlying circuitry. Behavioral and construct validity arguments regarding the accuracy of reinstatement as a model of human relapse to drug seeking are reviewed in great depth elsewhere (9, 10). The questions to be examined about reinstatement in this review are from a perspective of model utility in understanding the neurobiological underpinnings of drug-induced pathologies of motivation. We will consider the employment of the reinstatement paradigm in this regard as well in the development of pharmacotherapeutic treatments for addiction.
The Reinstatement Model
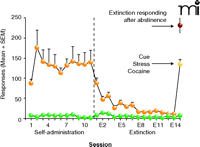
The reinstatement model was developed about twenty-five years ago (11). In its simplest form (Figure 1⇓), animals are first trained to self-administer an addictive drug in response to pressing a lever. The animal then undergoes a period of drug abstinence, during which they are typically re-exposed to the context where drug was previously delivered, with the sole difference being that lever pressing no longer results in drug delivery; “learning” under the extinction training paradigm is reflected by decreased rates of lever pressing. Subsequent to extinction training, lever pressing can be reinstated using three modalities of stimuli (each of which resonates well with a type of stimulus that causes human addicts to relapse): presentation of an environmental stimulus previously associated with drug administration; direct administration of the drug itself; and exposure to a stressor. Once lever pressing is reinstated, the investigator can ask a number of questions, the most useful of these falling into two categories: 1) What are the neurobiological underpinnings of reinstatement? and 2) Can reinstatement be inhibited by pharmacological or behavioral interventions that may be of predictive value in treating addiction in humans?
Development and Expression Phases of Addiction in an Animal Model. In the first phase of the paradigm, animals are trained for twelve days to self-administer cocaine by means of lever pressing (orange circles; drug delivery is accompanied by a cue—typically a light and/or a tone. In the second phase, animals either undergo "abstinence" (i.e., they are maintained in their home cages without exposure to the operant chamber) or "extinction training" during which no drug is administered in response to lever pressing (E1 –E14). At the end of this second phase, animals (regardless of whether "abstinent" [red] or "extinguished" [yellow]) are presented with the cue that had accompanied each drug infusion during self-administration, a mild stressor (typically foot shock), or the drug itself. Each of these stimuli reliably overcomes extinction training and the animals will press the lever even though no drug is delivered. This reinstatement of lever pressing is considered to be a bout of drug seeking. Note that, although reinstatement conditions mimic those of E1 for "reinstated" animals, the "abstinent" group responds to reinstatement far more greatly than do the extinction-trained animals. (Green represents responding on the control lever, which never delivers drug in response to lever pressing.)
Self-administration Training
As mentioned above, self-administration training of the reinstatement model has been the primary focus of study in addiction, and the reader is referred to many excellent reviews (12). For the purposes of this review, however, we would like to acknowledge recent discussions about the relative merits of giving animals short (e.g., one or two hours) versus longer (greater that four hours) daily periods of drug access. Much has been made of the fact that when animals are switched from short to long access, the rates of drug intake accelerate, akin to the increasing rates of drug use in the course of human addiction (7, 13). Moreover, animals trained according to long access paradigms will continue to self-administer drugs when experiencing greater adverse consequences than animals trained on shorter access to drugs, reminiscent of human addicts who use drugs in spite of adverse personal and sociological consequences (14). Although these characteristics lend strong face validity to the longer access paradigms, there has unfortunately been little research into underlying neurobiological mechanisms. More importantly, not all laboratories readily replicate accelerated drug intake in response to longer access (14–16). Moreover, given that acceleration in cocaine intake typically occurs only during the first hour of self-administration (17, 18), we would argue that acceleration of drug intake may simply result from the development of tolerance to the reinforcing effects of the drug, rather than from the induction of novel addiction-related neurobiological mechanisms [however, see (19, 20)].
Extinction Training
Extinction training has been employed in order to correct for the animal’s responsiveness to the context where drug was delivered. When reinstatement is induced (e.g., by cue, stress, or drug), an increase in lever pressing can readily be quantified. Recently, it has become clear that extinction training produces marked neurobiological changes in the brain that must be considered when studying the reinstatement of drug seeking (21). Specifically, the neurobiology of extinction training in fear conditioning paradigms has been studied in some detail, and the importance of amygdala regulation of the infralimbic cortex (i.e., the ventral prefrontal cortex [ventral PFC]) in extinguishing behavior has been highlighted. Thus, inactivation of the infralimbic cortex prevents extinction (22).
In recent studies from our laboratory, inactivation of the infralimbic cortex was shown both to inhibit within-session extinction learning, as well as disinhibit responding in animals whose lever pressing in a cocaine-paired context had been extinguished (J Peters and PW Kalivas; unpublished observations). Similar effects were observed after inactivation of the accumbens shell, suggesting that the ventral projection from ventral PFC to accumbens shell may form a circuit that inhibits drug seeking and that is strengthened by extinction training. In agreement with this notion, extinction is associated with increased content of the GluR1 subunit of the alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic (AMPA) glutamate receptor in the shell compartment of the nucleus accumbens (23). Thus, extinction training is an active process that has been shown to affect glutamate signaling in the nucleus accumbens and glutamatergic projections from the ventral PFC; it will undoubtedly prove to have many other influences on the neural circuitry underlying reinstatement behavior. Figure 2⇓ illustrates the hypothesized recruitment of glutamatergic projections from the infralimbic cortex to the accumbens shell in response to extinction training.
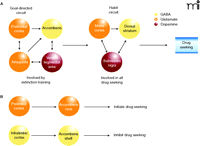
Brain Circuitry in the Re-instatement of Drug Seeking. A. Goal-directed and habit circuitries represent two levels of regulation of drug seeking. Lever pressing for drug becomes habitual during training, and if animals do not undergo extinction training, and are placed into the training environment after "abstinence" (see legend to Figure 1), behavior is regulated only by the classic corticostriatal habit circuitry. Extinction training, however, engages the second level of regulation—the cortico-amygdala-accumbens circuit, which modulates the habit circuitry via connections through the thalamus (not shown). [For reference, see (4, 42, 43).] B. Simplified rendering of the PFC-to-accumbens circuit that becomes involved by extinction training. The dorsal circuit from the prelimbic cortex to the accumbens core is necessary to initiate drug seeking in response to a cue, stress, or drug-priming injection in extinguished animals, whereas the ventral circuit from the infralimbic cortex to the accumbens shell appears to tonically inhibit drug seeking.
Reinstatement of Drug Seeking
Many studies over the last five years have used pharmacological inactivation of specific brain nuclei to determine the brain regions and circuits underlying reinstatement. Such studies afford an opportunity to determine how different stimuli (e.g., cue, drug, and stress), are integrated in the brain to elicit a common behavioral output (e.g., lever pressing or nose poking). Significantly, although diverse stimuli may involve distinct combinations of brain nuclei, overlapping components of a final common circuit appear to be recruited that involve glutamatergic input from the PFC to the nucleus accumbens (Figure 2A⇑) (4). Thus, stress involves regions of the extended amygdala and dopamine projections to the dorsal PFC (prelimbic cortex), whereas cues activate dopaminergic input to the amygdala that has glutamatergic projections to the prelimbic cortex and nucleus accumbens, and cocaine injections activate dopamine input directly into prelimbic cortex. More recently, other components have been added to the circuit shown in Figure 2A⇑, including a requirement for dopamine input to the shell of the accumbens and orexin input to the dopamine cells in the ventral tegmental area (24–26). Figure 2B⇑ schematizes a simplified circuit whereby extinction training and reinstatement by any of three environmental modalities involves the glutamatergic projections from the prefrontal cortex to the nucleus accumbens. The simplest implication of this model is that there exists a balance between the glutamatergic projections from the prelimbic and infralimbic cortices. The dorsal projection is recruited to drive drug-seeking behavior when a non-extinguished conditioned stimulus is presented to trigger relapse. The presence of this non-extinguished stimulus in an extinguished context presents a situation of conflict that requires the dorsal PFC for resolution (27). In the absence of such relapse-inducing stimuli, the infralimbic projection to the accumbens shell actively inhibits lever pressing. In fact, inactivation of the infralimbic cortex has been shown to reinstate non-drug goal-directed behaviors in rats (28), suggesting a universal role of this structure in response-inhibition. Importantly, a similar conclusion has been drawn from neuroimaging human addicts, where the rostral anterior cingulate cortex has been associated with drug craving (29). Moreover, studies of the brain circuits associated with motivation in a non-drug context (versus the inhibition of behavior) draw a similar conclusion by assigning a behavioral activating role to the anterior cingulate and an inhibiting role to the ventral orbital cortex (30, 31).
Abstinence without Extinction
A recent development in the study of brain circuits underlying reinstatement has been to examine the rates of lever pressing by animals that do not receive extinction training, but rather undergo extended abstinence (i.e., one to three weeks). When re-exposed to the drug context (i.e., the operant chamber) after a period of such abstinence, animals manifest rates of lever pressing in excess of those produced in animals that have received extinction training (Figure 1⇑). Using an inactivation strategy akin to the reinstatement paradigms described above for discerning drug-seeking circuitry, no glutamatergic projections to the nucleus accumbens, from either the PFC or the amygdala, proved critical for drug seeking under abstinence conditions, and inactivation of the dorsal striatum alone was found to inhibit drug seeking in the abstinent rat (32). Indeed, the dorsal striatum proves necessary for reinstatement of drug seeking by cue or drug after extinction training (33). Thus, dorsal striatal circuitry is necessary for any form of reinstatement, whereas dorsal PFC and allocortical circuits are obligatory in extinguished animals, but are apparently bypassed in abstinent animals lacking extinction training.
Goal-Directed versus Habit Circuitry in Addiction
Although the two overlap, the circuit involved in drug seeking after extinction training is more elaborate than the circuit underlying drug seeking in abstinence and involves dorsal prefrontal and allocortical brain regions (Figure 2⇑). The dorsal PFC and allocortical structures such as the amygdala are known to provide both cognitive and emotional context in behavioral regulation and decision making. Thus, it can be argued that drug seeking in both the abstinence and reinstatement paradigms requires involvement of habit circuitry that includes the classic striatothalamocortical circuitry (34), which is not surprising, given the compulsive nature of drug seeking in addicts. Indeed, this circuit is known to be activated in obsessive–compulsive disorders (35). The obligatory involvement of cortical and allocortical brain regions in the activity of drug seeking after extinction, however, may reflect a more regulated, less compulsive event. Indeed, the amount of lever pressing produced during a typical reinstatement paradigm is less than that produced after abstinence.
The enriched circuitry and the more regulated behavioral output (i.e., the reduction in lever pressing) involved in drug seeking after extinction training, as opposed to abstinence alone, may be relevant to addiction in human. In neuroimaging studies, the induction of craving by drug-associated cues activates brain circuitry reminiscent of reinstated drug seeking in animals that have undergone extinction training (29). The pattern of brain activation argues that, at the time of testing, addicts invoke both cognitive and emotional processes associated with craving. Of course, the addict in the laboratory setting cannot “relapse” into compulsive drug use. Thus, it remains unknown if addicts in a less cognitively and emotionally demanding environment than a laboratory setting will indeed involve cortical and allocortical brain regions in the process of relapsing. Nonetheless, the extant data argue that extinction training in rodents involves cognitive and emotional circuitry reflective of drug cravings in humans.
Cognitive Enhancers: Pharmacological Modulation in Animal Models of Relapse
As outlined above, extinction training results in more regulated, reduced amounts of drug seeking, because it involves inhibitory PFC areas. Activation of infralimbic or ventral orbital cortices, which are PFC areas in rats and humans, respectively, are known to provide behavioral inhibition (36, 37). One of the most replicated data sets in human neuroimaging studies is that addiction is associated with an overall reduction in basal metabolic activity in the PFC, and recent studies indicate that drug addiction is associated with cognitive deficits. These cognitive deficits are thought to contribute to the difficulty experienced by addicts in regulating drug-seeking behaviors, and thus, “hypofrontality” may offer a potential pharmacotherapeutic target. Specifically, pharmacological restoration of PFC regulation of drug-associated habit circuitry may permit addicts to better regulate drug seeking and more readily respond to cognitive behavioral therapeutic interventions. Along these lines, reinstatement animal models and clinical evidence currently suggest that drugs promoting cognitive function may be useful in treating addiction. Both modafinil and N-acetylcysteine affect glutamate transmission and have been examined in cocaine addiction (38, 39). Modafinil reportedly blunts cocaine euphoria and may have an effect on psychosocial treatment of cocaine dependence. N-acetylcysteine, which targets glutamate homeostasis by activating cystine-glutamate exchange (40), appears to blunt cocaine cue–induced activation of the anterior cingulate cortex in cocaine addicts.
Conclusions
The reinstatement animal model of drug seeking has revealed distinct but overlapping brain circuits that integrate different modalities of stimuli that regulate relapse in human addicts. The model has recently been used to compare drug seeking in animals that have undergone abstinence (without extinction training) with animals that have experienced extinction training. These comparisons have revealed that extinction training engages cortical and allocortical circuitry to modulate striatal habit circuitry, resulting in more regulated drug seeking as indicated by reduced lever pressing.
In addicts, cognitive behavioral therapy has proven somewhat useful in preventing relapse, presumably by increasing cognitive regulation of striatal habit circuitry. The utility of improving cognitive regulation of drug seeking is indicated by neuroimaging and cognitive testing, which show that addiction is associated with reduced PFC basal metabolic activity and with deficits in decision-making and in the inhibition of behaviors (29, 41). Thus, a proposed pharmacotherapeutic strategy is to fortify cognitive regulation of behavior. Given that corticostriatal projections are glutamatergic, compounds regulating glutamate transmission have been evaluated in reinstatement animal models and in early clinical trials. Although definitive double blind studies evaluating these drugs in recidivism are currently underway, the data to date can be considered encouraging.
Acknowledgments
Some of the research described in this review was supported by National Institute of Drug Abuse grants DA05369 (PWK), DA12513 (PWK), DA03906 (PWK), DA021975 (LK) and DA018486 (JP). doi:10.1124/mi.6.6.7
- © American Society for Pharmacology and Experimental Theraputics 2006