
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
Cyclic Nucleotide Signaling Mechanisms in Trypanosomes: Possible Targets for Therapeutic Agents
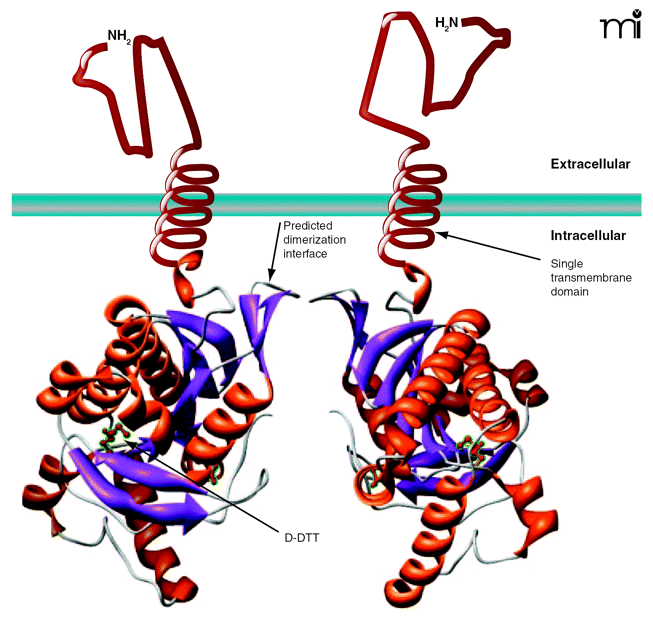
Representation ofT. bruceiadenylyl cyclases (ACs). The adenylyl cyclases (ACs) found in T. brucei contain a variable extracellular domain, a single transmembrane domain, and a catalytic domain. The catalytic domain shown is that of the GRESAG4.1, which can dimerize, leading to the activation of these enzymes. Development of synthetic ligands that force the dimerization of these ACs would provide valuable research tools to study the role of these enzymes in trypanosomes and may have possible therapeutic uses. Additionally, the crystal structure shows a molecule of (2S,3S)-1,4-dimercapto-2,3-butanediol (D-DTT) bound at a site within the catalytic domain. This site might be targeted through the development of agonists or antagonists designed to bind the AC and modulate its activity.


