
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
Cyclic Nucleotide Signaling Mechanisms in Trypanosomes: Possible Targets for Therapeutic Agents
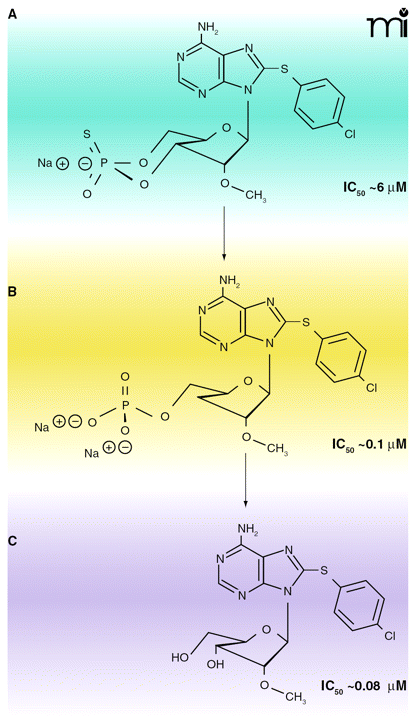
Hydrolysis products of cAMP analogs in trypanosomes have anti-proliferative activity. Membrane-permeable cAMP analogs are hydrolyzed by T. brucei PDEs, and the hydrolysis products (adenosine and 5′-AMP forms) show stronger anti-proliferative activities than the cAMP analogs themselves. These hydrolyzed products cause the conversion of long, slender forms of T. brucei to short, stumpy-like forms. Shown here are the structures and IC50 values for the antiproliferative effects of 8-pCPT-2′-O-Me-cAMP (A) and its hydrolysis products, 8-pCPT-2′-O-Me-5′-AMP and 8-pCPT-2′-O-Me-ado (B and C, respectively). Some of these cell-permeable adenosine analogs could be further developed as potential anti-trypanosomal agents. Data from (82). Used by permission. ©2006, National Academy of Sciences Press.


