
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
Diversity of the Neurotoxic Conus Peptides
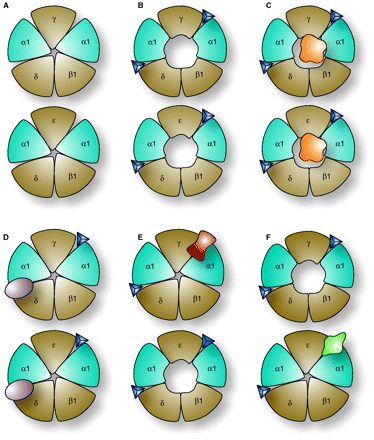
Alternative target sites of acetylcholine receptors (nAChRs). Each panel (A to F) shows two muscle nAChRs; the top receptor is the “fetal” subtype, with a γ subunit, and the bottom is the “adult” subtype, with an ε subunit. In the absence of acetylcholine (blue triangles) receptors are closed. When two molecules of acetylcholine bind (as shown in B), a transition from a closed to an open state occurs (compare A to B). Panels C, D, E, and F show effects of different conotoxins. Panel C demonstrates one possible mechanism of non-competitive block. In this example, the toxin (orange globular geometry) occludes ion conductance through the channel pore. Panels D, E, and F show competitive antagonists targeted to different pharmacological sites. In panel D, a conotoxin (purple) highly specific for α1δ is shown; this inhibits both fetal and adult subtypes. In E, a conotoxin (red) specific for the α1γ interface binds the fetal receptor, but not the adult subtype (which can therefore open normally). The converse is true in F, in which the conotoxin (green) is specific for α1ε; this does not inhibit the fetal subtype, but prevents acetylcholine from binding one of the two ligand sites in the adult subtype. Thus, to inhibit both receptors subtypes, either a single peptide with the specificity shown in D, or two different peptides with the specificities shown in E and F are required. In fish-hunting Conus venoms, there are usually two or more peptides targeting these muscle receptors; a venom with both the peptides shown in C and D would inhibit these receptors more efficiently than venoms with only one type of nAChR antagonist.


