
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
BEYOND AMYLOID: The Next Generation of Alzheimer’s Disease Therapeutics
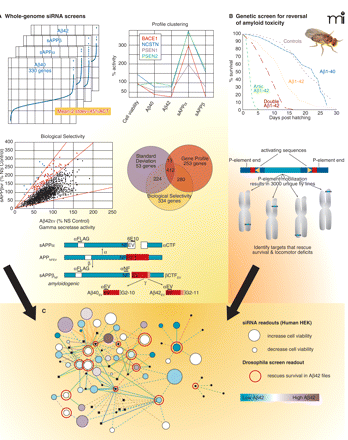
The convergence of two distinct genomic screens that together identify genes that regulate APP processing and amyloid neurotoxicity. A) The development of inhibitors APP proteolysis (i.e., inhibitors of γ-secretase) for the treatment of Alzheimer’s disease has been hampered by mechanism-based toxicities. To identify novel regulators/modulators of APP processing, a whole-genome siRNA screen was conducted in HEK293 cells expressing the NEFV mutant of APP—an engineered mutation that facilitates cleavage and assay of the Aβ42 fragment. Viability and multiple endpoints of APP processing were monitored, including the production of Aβ40, Aβ42, soluble APPβ (sAPPβ), and soluble APPα (sAPPα). Hits were selected based on high-throughput results within two standard deviations from the mean as well as by patterns of inhibition reflecting BACE-like activity (i.e., decrease in Aβ40, Aβ42, and sAPPβ and an increase in sAPPα). [See (74).] B) The expression of human Aβ42 peptides in fruit flies results in decreased lifespan, locomotor deficits, and rough-eye phenotype. These characteristics are not observed in flies expressing the benign human Aβ40 peptide and are exacerbated in flies expressing either a double dose of Aβ42 or aggregation-prone forms of Aβ42. A p-element genetic screen was performed to identify genes that, when overexpressed or disrupted, would rescue flies that express an aggregation-prone form of Aβ42. Several genes were identified that rescue Aβ42-mediated toxicity and are currently under investigation. [See (80).] C) Exploring protein-protein interaction networks reveal a convergence of the data sets indicating that pathways that regulate APP processing may also play a role in mitigating Aβ42 related neurodegeneration.


