
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
The See-saw of Differentiation: Tipping the Chromatin Balance
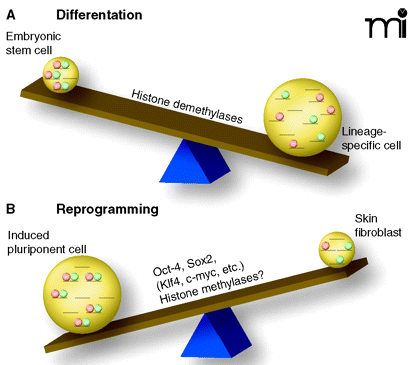
The see-saw of differentiation. Chromatin patterns of histone modification (i.e., acetylation, methylation, etc.) determine accessibility for transcription factors and, thus, direct tissue-specific transcription. A recent study identified a novel pattern of histone 3 methylation that is unique for embryonic stem cells (A). The epigenetic mark, termed bivalent [because it includes H3K4 methylation (green circles) as well as H3K27 methylation (pink circles)] can be found at the transcriptional start sites of many developmental genes (1, 2). These sites are poised for transcription in stem cells and resolve upon differentiation in either H3K4 or H3K27 methylation, associated with transcriptional activation or repression, respectively. Skin fibroblast cultures (upon introduction of four transcription factors) can revert into stem cells (iPS, induced pluripotent cells) (17). (B) Upon de-differentiation, the bivalent chromatin pattern is re-established. Identifying the key molecular mechanisms that are responsible for making the switch between pluripotency and lineage specificity opens the possibility of manipulating the see-saw of differentiation for regenerative medicine.


