Revealing the Potential of Intranasally Administered Orexin A (Hypocretin-1)
Orexin A (hypocretin-1) is a neuropeptide predominantly expressed in neurons of the lateral hypothalamic and perifornical areas with vast projections to the cerebral cortex, limbic system, locus coeruleus, posterior hypothalamus, thalamus and brainstem (1) (Figure 1⇓). Currently, orexin A is regarded as an essential mediator between energy homeostasis and the regulation of sleep–wake rhythmicity. Intracerebroventricular (icv) administration of orexin A into the central nervous system (CNS) acutely stimulates feeding in rodents, especially at times of day when regular food intake is at its lowest (2, 3); however, orexin A does not induce permanent weight gain because compensatory hypophagia develops with chronic orexin A treatment (3). These and similar observations have challenged the view of an immediate effect of orexin A on feeding. It is indeed more plausible that this effect arises from an interaction between circuitries regulating food intake on the one hand and sleep/wakefulness and global central nervous arousal on the other hand. Orexin A neurons are inactive during sleep and, vice versa, active during wake-fulness (4, 5). Fittingly, in nocturnal rodents (6) as well as in diurnal squirrel monkeys (7), concentrations of orexin A in the cerebrospinal fluid (CSF) are at their lowest at the time of awakening and rise linearly until reaching maximal concentrations before a sleep-associated decrease occurs. Sleep deprivation further increases concentrations of orexin A in CSF (7), and central nervous administration of orexin A increases wakefulness while suppressing rapid eye movement (REM) sleep (8), supporting the notion that orexin A signaling is essential for establishing a constant level of alertness.
The projection fields of orexin A neurons in humans appear to overlap those in rodents, where most experimental work has been performed (9). In humans, the hypocretin system is deficient in most cases of narcolepsy, a disorder of the sleep–wake cycle characterized by chronic daytime sleepiness, cataplexy (i.e., sudden loss of muscle tone triggered by strong emotions), sleep paralysis, hypnagogic hallucinations, and a decrease in the time period between falling asleep and the onset of REM sleep (10). Accordingly, a mutation in the hypocretin-2 receptor (orexin receptor 2) gene is a cause of canine narcolepsy (11), and a null mutation of the gene encoding the two known orexin A peptides produces a narcoleptic phenotype in mice (12). In contrast, there is some evidence that CSF concentrations are elevated in untreated patients suffering from early-onset restless legs syndrome (RLS) (13). RLS is characterized by the subjective, unpleasant experience of restlessness that becomes predominant in the evening and by focal acathisia (i.e., inability to sit still or remain motionless), resulting in severely disturbed nocturnal sleep with concomitant periodic limb movements (14, 15). Daytime sleepiness, however, is a rare problem in those patients. Although the pathophysiology of RLS is attributed to malfunctions of the dopaminergic system and/or iron metabolism (16), the “wake-promoting” effect of an over-active orexin system may contribute to RLS-related symptoms and, in particular, induce the constant level of daytime alertness that patients display in spite of poor nocturnal sleep quality.
Given the involvement of the orexin system in the pathophysiology of sleep–wake disorders, targeting central nervous orexin pathways might be a valuable therapeutic option in the treatment of such disorders. Indeed, pharmacological blockade of orexin A signaling by oral administration of a dual antagonist that is active at both the orexin 1 and orexin 2 receptor during the active period of the circadian sleep–wake cycle induces indicators of somnolence in rats, dogs, and also in humans (17). It must be noted that most research in animals on the behavioral effects of orexin A has relied on icv administration of the compound, with early reports pointing out that systematically administered orexin A might not cross the blood-brain barrier (BBB) in amounts high enough to alter brain functions (18). Studies in mice, however, have indicated that intravenously injected orexin A rapidly reaches the brain parenchyma in intact form and that this effect is not saturable (19). Accordingly, in narcoleptic dogs, systemically administered orexin A increased the level of activity, prolonged waking periods, decreased REM sleep, and induced a dose dependent reduction in cataplexy (20). Nonetheless, comparable experiments in dogs failed to yield equally promising results (21), casting doubt on the efficacy of systematically administered orexin A to affect brain circuitries of sleep–wake regulation.
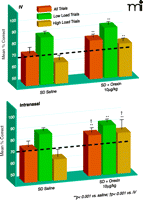
Recently, elegant experiments by Deadwyler et al. (22) on the effect of orexin A in sleep-deprived rhesus monkeys have further fuelled the ongoing discussion about the central nervous administration of the compound. In this study, two methods of orexin A delivery were compared: intravenous injections and a new approach using the intranasal pathway to deliver the peptide to the brain (23). To examine alerting effects of orexin A, rhesus monkeys, after sleep-deprivation of thirty to thirty-six hours, were treated with orexin A immediately before they were tested on a delayed match-to-sample (DMS) memory task (Box 1). Both intravenously administered orexin A (2.5–10.0 μg/kg) as well as intranasal orexin A (1.0 μg/kg) improved performance on this task. Intriguingly, however, intranasal administration of the peptide was significantly more effective than the highest dose of intravenous orexin A (Figure 2⇓). Intranasal, but not intravenous, administration of orexin A returned general performance on the DMS task to normal levels. It is also to note that the 10.0 μg/ kg dose of intravenous orexin A actually impaired performance on the same task in non-sleep-deprived, alert monkeys, whereas intranasal orexin A had no effect in non-sleep-deprived animals. Enhanced performance after orexin A treatment in sleep-deprived animals was associated with the reversal of changes in cerebral glucose metabolism that had been induced by sleep deprivation in brain regions including the dorsolateral prefrontal cortex, dorsal striatum, medial temporal lobe, and thalamus. Again, the effects of intranasal orexin A on brain metabolic activity were more pronounced than those of intravenous orexin A. Superiority of intra-nasal in comparison to intravenous orexin A administration has likewise been inferred from studies on peptide binding to orexin A receptors in the brain of anesthetized mice (24). Deadwyler and colleagues (22) conclude that their findings provide evidence for the effectiveness of intranasal orexin A in alleviating cognitive deficits induced by sleep loss and that intranasal orexin A is likely to overcome the limitations of the use of intravenous orexin A, including, but not limited to, lack of bioavailability and drastically reduced potency in comparison to central administration.
The Delayed Match-to-Sample (DMS) Memory Task
The DMS is a multi-image short-term memory task that sensitively detects cognitive defects produced by sleep deprivation (39). The DMS trials require animals to initiate the presentation of a sample image on a display. After a delay phase ranging from 1–90 seconds during which the screen is blanked, the sample image is presented simultaneously with 1–8 randomly chosen “non-match” distracter images. The monkeys are then required to place the cursor in the sample image to be rewarded and terminate the trial. Long delay (60–90 sec) trials with more distracter images (6–8) are defined as high-cognitive-load trials, whereas trials with short delays (1–15 sec) and few distracter images (1–3) constitute low-cognitive-load trials.
There are some caveats to far-reaching conclusions at this point. Although sleep-deprived animals clearly benefited from orexin A administration, the highest dose of intravenously administered orexin A impaired cognitive performance in alert animals. The authors reason that the effects of orexin A in the waking state may differ from those obtained after sleep deprivation because of different levels of orexin A receptor activation (20, 25), and this might also be the case with higher doses of intranasally applied orexin A. Also, with some experimental evidence pointing towards possible species-dependency of the physiological role of orexin A (26, 27), effects in humans may be at even greater variance with findings in (sleep-deprived) rhesus monkeys, and this possibility may in particular apply to patients suffering from orexin A dysfunctions. Furthermore, the promising results obtained after acute orexin A administration might markedly weaken after long-term exposure to the compound.
The specific advantage of intranasal administration of neuropeptides to the CNS derives from the fact that central nervous circuitries can be directly targeted via intranasal administration while avoiding the pitfalls of systemic administration (i.e., possible peripheral side effects). In humans, intranasal administration of peptides like insulin allows direct access to the CSF compartment within thirty minutes, bypassing uptake into the bloodstream (23). These observations and the results brought forward by Deadwyler and colleagues (22) are in line with findings in animals where intranasal administration of peptides and of larger molecules led to an accumulation of the substances in brain tissue (28) [for review, see (29)]. Given that the intraneuronal transport of neuropeptides after intranasal administration would face proteolytic obstacles as a result of lysosomal degradation and, thus, would take several hours for the substance to reach the olfactory bulb, it is much more plausible to assume that the peptide molecules, via extraneuronal transport, pass through intercellular clefts in the olfactory epithelium to diffuse into the subarachnoidal space (29, 30). We have been able to demonstrate in humans that by intranasal administration of neuropeptides brain functions can be effectively modulated. For instance, long-term intranasal administration of insulin reduces body fat stores (31) while improving memory functions (32), suggesting that intranasal insulin is a viable approach to counteract memory disorders like Alzheimer’s disease (33) and also to reduce the cognitive impairments that obesity is suspected to entail (34). The Deadwyler et al. study likewise bodes well for the use of intranasally administered orexin A and orexin A analogs in the therapy of disorders such as narcolepsy that is currently treated purely symptomatically with dopamine agonists and tricyclic antidepressants (35). Intranasal devices are very easy to use, thus ensuring good compliance and safety, which would be two important advantages over intravenous administration when a drug must be taken every day. Also, central nervous mechanisms of disorders like type 2 diabetes mellitus that are traditionally regarded to originate solely from systemic pathophysiologies are only on the verge of being elucidated (36, 37), and intranasally administered therapeutic agents might one day join peripherally applied drugs for treating those diseases. Thus, future studies and clinical trials will have to examine if the intranasal pathway can meet the great expectations raised by the promising results of basic research (38).
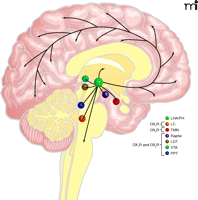
Main projections of orexin neurons. This figure summarizes orexinergic projections in the human brain as predicted from rodent studies. Circles show regions with strong expression of orexin receptors type 1 and type 2 (OX1R and OX2R, respectively) and dense orexinergic projections. Orexin neurons originating in the lateral hypothalamic area (LHA) and posterior hypothalamus (PH) regulate sleep and wakefulness and the maintenance of arousal by sending excitatory projections to the entire CNS, excluding the cerebellum, with particularly dense projections to monoaminergic and cholinergic nuclei in the brain stem and hypothalamic regions, including the locus coeruleus (LC, containing noradrenaline), tuberomammillary nucleus (TMN, containing histamine), raphe nuclei (Raphe, containing serotonin) and laterodorsal/pedunclopontine tegmental nuclei (LDT/PPT), containing acetylcholine). Orexin neurons also have links with the reward system through the ventral tegmental area (VTA, containing dopamine) and with the hypothalamic nuclei that stimulate feeding behavior. Figure from (1). Used with permission.
Comparing routes of orexin A administration for efficacy in reversing sleep deprivation deficits. Comparison of the efficacy of intravenous (IV; top panel) and intranasal orexin A (bottom panel) regarding the reversal of sleep deprivation (SD)-induced deterioration of performance in the delayed match to sample (DMS) short-term memory task [from (21)]. Eight adult male rhesus monkeys weighing 8.0–11.0 kg were examined under normal, alert conditions and after 30–36 hours of SD. Effects of the two delivery methods on mean percentage correct (+/−SEM) performance relative to performance levels after SD and saline administration (dotted line) are indicated. Overall performance is shown for all trials (orange bars) and for trials of low cognitive load [green bars; trials with short inter-stimuli delays (1–15 s) and 1–3 distracter images] and high cognitive load [dark gold bars; long duration (60–90 s) trials with 6–8 distracter images]. **p<0.001, difference relative to each respective saline SD condition; †p<0.001, difference between intravenous versus intranasal orexin A in SD condition. Used with permission (22).
- © American Society for Pharmacology and Experimental Theraputics 2008
References

Jan Born, PhD, is head of the Department of Neuroendocrinology at the University of Lübeck. He obtained PhDs in Psychology at the University of Tübingen and in Physiology at the University of Ulm (Germany). After a stay as research fellow in the Department of Biological Psychology at the State University of New York at Stony Brook, he worked as post doc and assistant professor in the Department of Physiology at the University of Ulm. In 1989, he was appointed full professor of Physiological Psychology at the University of Bamberg. In 1999, he joined the Department of Neuroendocrinology at the University of Lübeck. He is a Member of the Berlin-Brandenburgische Academy of Science. Jan Born’s primary research interests are in the dynamics of memory formation. He is speaker of the collaborative research center “Plasticity and Sleep,” founded in 2005 by the German Science Foundation at the Universities of Lübeck and Kiel.

Manfred Hallschmid, PhD, is assistant professor at the Department of Neuroendocrinology at the University of Lübeck. He obtained a diploma in psychology at the University of Bamberg in 1999. In 2005, he obtained a doctorate degree in psychology/human biology at the University of Lübeck. He has received research awards by the German Society of Psychology, the German Society of Endocrinology, and the German Diabetes Society in 2002, 2003, and 2005, respectively. He is project leader in the Lübeck-based clinical research group “Selfish Brain” founded by the German Science Foundation. Manfred Hallschmid’s primary research area is the central nervous regulation of food intake and body weight in humans with a particular focus on the intransal administration of neuropeptides. He studies the crosstalk between nutritional and cognitive factors and the relationship between sleep and metabolism. E-mail: hallsch-mid{at}kfg.uni-luebeck.de; fax: +49-451+5003640.