
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
Is a Step Backwards in S-Phase-Targeted Chemotherapy a Step Forward?
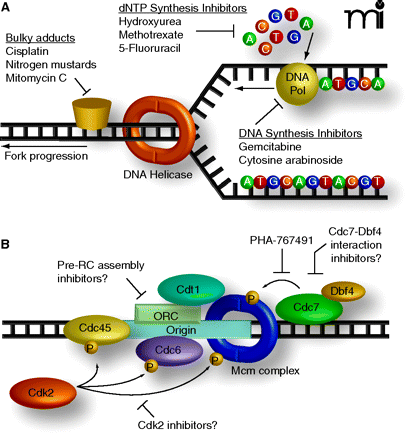
Current and hypothetical targets for antineoplastic therapy targeting DNA synthesis. A. FDA-approved cancer drugs that target S-phase cells disrupt the replication elongation stage, during which the replication fork is already formed. Collision of the replication fork with DNA adducts can induce DNA strand breaks, which activate the S-phase checkpoint. Similarly, the progression speed of the fork is monitored by the transducer kinase ATR. If the fork is destabilized or slowed significantly, ATR mediates checkpoint activation. Thus, the current chemotherapeutics activate a replication fork-dependent checkpoint. B. PHA-767491 targets the kinase Cdc7, which is required for the replication initiation phase. In the initiation phase, the replication fork is not yet formed, thus, interfering with this step of DNA synthesis does not elicit a checkpoint response. The efficacy of PHA-767-491 in multiple cellular and animal models, combined with its apparent selectivity for cancerous cells suggest that targeting the replication initiation machinery may be a novel and effective method for chemotherapy.


