Does Targeting the Lipophilic Milieu Provide Advantages for an Endothelin Antagonist?
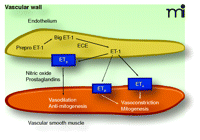
The endothelin (ET) system comprises three 21-amino acid peptides (ET-1, ET-2, and ET-3). Of these, only ET-1 appears to play an important physiological and pathophysiological role in the cardiovascular system. ET-1 acts mainly in a paracrine fashion and is an extremely potent and long-lasting vasoconstrictor. ET-1 is released principally from endothelial cells and is initially synthesized as Prepro-ET-1, a 203-amino acid peptide, which is cleaved into big ET-1, a 38-amino acid peptide. Big ET-1 is in turn cleaved into its functional form (ET-1) by endothelin converting enzyme-1 (1–3). As shown in Figure 1⇓, the hemodynamic and proliferative effects of ET-1 are mediated through two receptor subtypes: ETA receptors, located on vascular smooth muscle cells (VSMCs), are responsible for the vasoconstricting effects of ET-1; and ETB receptors, located on vascular endothelial cells, are responsible for the vasodilating effects of ET-1. Vasodilation is mediated by endothelial-derived relaxing factors including nitric oxide (NO) (4–6). A subpopulation of ETB receptors is also found on VSMCs and induces vasoconstriction when activated (7). In addition to its vasoconstrictor and pressor effects, ET-1 can induce hypertrophy and hyperplasia of cardiomyocytes (8), stimulate fibroblast proliferation (9), increase extracellular matrix production (10), and stimulate a pro-inflammatory phenotype (11). ET-1 also alters the generation of vasomediators other than NO, including prostacyclin and platelet-activating factors (7). Circulating plasma ET-1 concentrations are elevated in atherosclerosis, arterial hypertension, heart failure, and pulmonary arterial hypertension (PAH) as compared to those concentrations observed in the normal state (12). ET-1 plasma concentrations also correlate inversely with reduced pulmonary function (13) and survival in patients with PAH (14).
The actions of ET-1 led to a wide-spread interest in endothelin antagonists for the treatment of various cardiovascular and other disorders that has been sustained since the initial discovery of a poorly specific endothelin receptor antagonist (15) and the generation of compounds based on the ETA receptor antagonist BE-18257A, isolated from Streptomyces misakiensis (16). These peptide inhibitors led to the initiation of small-molecule programs at most of the major pharmaceutical companies. We now have a wide range of compounds that are either selective for the ETA receptor, or antagonize both ETA and ETB receptors, also known as combined, or dual antagonists. The promise of therapeutic utility of endothelin antagonists has been realized with several drugs that have proven efficacy in PAH (17–19). Clinical trials continue for a range of targets such as resistant hypertension, chronic kidney disease, and prostate cancer (http://clinicaltrials.gov). One of the most significant developments during the early years of development of these compounds was the founding of a small Swiss biotech company, Actelion Pharmaceuticals Ltd., which developed the first US Food and Drug Administration-approved endothelin antagonist, bosentan (Tracleer®), a dual antagonist used clinically for the treatment of PAH (20).
Although the efficacy of bosentan and other endothelin antagonists for the treatment of PAH, including selective ETA antagonists (18), is well established, efforts continue to improve efficacy and utility of this class of compounds. Indeed, there are ongoing debates of the relative merits of ETA receptor–specific antagonism compared to combined–receptor antagonism. On the one hand, activation of ETB receptors on endothelial cells enhances NO production (21), which would argue in favor of utilizing selective ETA receptor antagonists. The STRIDE (Sitaxsentan To Relieve Impaired Exercise) trials have demonstrated that the ETA receptor antagonist sitaxsentan, at a dose of 100 mg/day orally resulted in significant improvements in both 6-minute-walk distance and heart health assessment (New York Heart Association functional class), as well as improvements in pulmonary vascular resistance and cardiac index (18). There is, however, also a subset of ETB receptors located on VSMCs that cause vasoconstriction when activated. Further, at least in animal models of PAH, there can be a longitudinal change in endothelin receptor density whereby early induced expression of ETA and attenuated expression of ETB receptors is followed by a later induction of ETB receptor expression on VSMCs (22). Thus, the use of a combined–receptor antagonist could attenuate the harmful effects of endothelin signaling in vascular smooth muscle from both receptors. However, ETA receptor–selective and dual antagonists suffer notable limitations, including elevation of liver enzymes (23) and narrow therapeutic windows (18). Thus, the development of an optimal ET receptor antagonist is ongoing.
Iglarz and colleagues from Actelion have recently published a study describing a new dual endothelin antagonist, macitentan. This antagonist was designed for tissue targeting by improving its lipophilicity (24). These investigators are to be applauded for the novelty of their compound, but there remain many questions requiring answers before macitentan can be deemed to have any significant clinical benefit beyond that of existing ET receptor antagonists. Macitentan, also called Actelion-1 or ACT-064992, was discovered through tailored screening to have a high partition coefficient for lipid versus aqueous solutions. A compound that partitions to lipid environments would be expected to remain in local tissue environments, providing therapeutic advantages, such as a prolonged functional half-life in vivo, or as Iglarz and colleagues propose, more effective targeting of the autocrine-paracrine endothelin system. Another issue that will need to be investigated is whether the lipophilicity of macitentan enhances penetration of the blood-brain barrier. Although the central effects of endothelin are still poorly understood, there may be clinical benefits to targeting the brain in terms of reducing sympathetic nerve activity (25), ameliorating the harmful effects of endothelin in stroke (26) and dementia (27, 28).
The study by Iglarz et al. clearly establishes macitentan and its major metabolite, ACT-132577, as having high affinity for both ETA and ETB receptors in a cell-expression system as well as being fairly potent at inhibiting ET-1-induced increases in intracellular Ca2+ (24). Furthermore, they establish in vivo efficacy in lowering blood pressure in mineralocorticoid (DOCA)-salt hypertensive rats, improving survival in monocrotaline-induced pulmonary hypertension, and reducing proteinuria in streptozotocin-induced diabetes––disease models that have shown benefit from the use of selective ETA and dual ETA-ETB antagonists (7). One of the hallmarks of ETB receptor blockade in vivo is elevation of plasma ET-1 concentrations. Because macitentan increases plasma ET-1 at a 10-fold lower dose compared to that of bosentan, the authors conclude that macitentan has greater in vivo potency relative to bosentan (24). Caution should be used in over-interpreting these data, however, as plasma ET-1 was measured at only one time-point following acute oral administration of the two drugs. The authors reported the more physiologically relevant finding that acute administration of macitentan or bosentan produced similar reductions in blood pressure of DOCA-salt hypertensive rats but that this was more prolonged and achieved at a 10-fold lower dose with macitentan. Although Iglarz et al. suggest that the greater potency of macitentan relative to bosentan is due to macitentan’s lipophilic nature, the authors will need to provide direct in vitro comparison of binding affinity of macitentan and bosentan as well as data demonstrating the concentrations of the drugs achieved in the plasma. Thus, one cannot exclude the possibility that the difference in potency in vivo between macitentan and bosentan may be attributable to an improved binding affinity or oral bioavailability and not due to partitioning into tissues. Importantly, these investigators will need to provide quantitative information that demonstrates actual accumulation or targeting in tissues compared to a compound with more hydrophilic properties.
One attractive feature in developing an endothelin antagonist that targets or accumulates in tissues is that ET-1 is an autocrine-paracrine factor. In this sense, the characteristics of macitentan to partition to a lipid environment would provide an advantage. A limitation to the Iglarz et al. study is that these investigators did not report tissue concentrations of macitentan or its major, more long-lived metabolite ACT-132577, either following acute administration or chronic treatment. It would have been particularly informative to compare tissue concentrations of these compounds and of bosentan in the disease models studied, especially in the relevant target organs (lungs for PAH, kidney for diabetes). The question remains whether the characteristic of macitentan to accumulate in tissues provides any improvement over a similarly potent compound in the in vivo models. Also, it also remains to be demonstrated whether a lipid-targeted compound actually provides prolonged and more effective blockade of ET receptors relative to a less lipophilic compound with equal receptor binding affinity. The extracellular milieu in which the drug must first enter is aqueous; furthermore, endothelin receptors are presumed to be extracellular and are exposed to this aqueous environment. It is not clear where in the body a lipophilic endothelin antagonist would partition itself. These types of analyses will be required in order to better understand whether this is a more effective approach for endothelin blockade.
Another topic that will need to be addressed in future studies is whether prolonged tissue accumulation would actually represent an advantage particularly in the event of the development of unwanted side effects. Liver toxicity, as determined by elevated liver aminotransferases, seems to be a class effect of endothelin receptor antagonists (23). ETA receptor–selective antagonists may exhibit less liver toxicity, but are frequently associated with increased incidence of peripheral edema and headache (23). Given the much lower doses of macitentan compared to bosentan (30 mg/kg vs 300 mg/kg, respectively) required to obtain a significant effect on the PAH phenotype, it is possible that low-dose macitentan could reduce the overall liver toxicity associated with the use of endothelin receptor antagonists, although this remains to be determined. In addition, while there have been no reports of rebound effects of withdrawing either selective or dual antagonists, a compound that is more likely to increase plasma ET-1 concentrations might have a greater likelihood of ET-1-induced effects once drug treatment is withdrawn. This is a possibility that would need to be monitored in future investigations.
Macitentan represents a dual endothelin receptor antagonist by virtue of its ability to block both ETA and ETB receptors simultaneously. Iglarz et al. argue that dual antagonism represents a significant therapeutic advantage because vasoconstriction-promoting ETB receptors are present on VSMCs and contribute to pulmonary hypertension and other vascular disorders. However, as activation of ETB receptors on endothelial cells is known to enhance NO generation (21), macitentan could, in theory, further attenuate NO signaling, thus exacerbating a hypertensive phenotype. Moreover, ETB receptors on tubular elements within the kidney play a critical role in the control of sodium excretion, and interference with ETB receptor function promotes salt-sensitive hypertension (29, 30). Thus one could argue that ETA receptor blockade may be more advantageous than combined ETA and ETB receptor blockade. Clearly, both dual and selective antagonists have proven clinical efficacy in several applications (23) and so one could argue that it does not matter whether the ETB receptor is blocked or not. Unfortunately, there are very few studies that directly compare dual versus selective antagonists, especially in humans, and those that have, provide data in support of both arguments, for example in monocrotaline-induced pulmonary hypertension (31, 32). Furthermore, and as discussed above, there are many circumstances where receptor expression is altered by disease, making these comparisons more important. Iglarz and colleagues argue that ETB receptors mediate the harmful effects of ET-1 in pathology, but this may only apply to specific indications. In the case of diabetic nephropathy, for example, animal studies have shown that the absence of ETB function can worsen the development of renal dysfunction and injury (33). Another problem is the lack of direct comparison of selective versus non-selective antagonists in clinical trials. Therefore, it will be left to academic investigators to undertake studies comparing the efficacy of dual versus selective antagonists, which may be difficult without industry support.
In conclusion, there is a clear need for more effective treatments for devastating diseases such as pulmonary hypertension, cancer, and chronic kidney disease. Targeting the endothelin system has already proven to offer benefit in these diseases, but there is still much room for improvement in this class of drugs. The approach of designing endothelin receptor antagonists to more effectively target this predominantly autocrine-paracrine system may lead to therapeutic advances. Thus, we await future studies with interest that will examine tissue accumulation and distribution, toxicity and side-effect profiles, and efficacy of more lipophilic, tissue-targeted endothelin receptor antagonists.
Fundamental mechanisms of ET-1 synthesis and receptor-specific actions in the vascular wall. Endothelin-1 (ET-1) synthesis begins with the initial gene product Prepro-ET-1 and is processed by series of proteolytic cleavages to form the immediate precursor, Big ET-1. Big ET-1 is cleaved by ECE to become ET-1. Big ET-1 is stored and released along with the more potent peptide, ET-1. ET-1, in turn, acts in an autocrine fashion to activate ETB receptors in endothelial cells to release vasorelaxing factors such as nitric oxide and prostaglandins. These factors have well-characterized actions to produce vasorelaxation and inhibit growth and proliferation of vascular smooth muscle. On vascular smooth muscle, the primary target is ETA receptors, which produce vasoconstriction along with mitogenic effects. In some vascular beds and in some pathological conditions, ETB receptors on vascular smooth muscle mimic the actions of the ETA receptor. However, little is known about the conditions in which these receptors are physiologically or pathophysiologically significant. ECE, endothelin–converting enzyme.
- © American Society for Pharmacology and Experimental Theraputics 2009
References
Erika Boesen, PhD, received her doctorate degree from the Department of Physiology at Monash University, Melbourne, Australia. She is currently an Assistant Research Scientist in the Vascular Biology Center and Department of Physiology of the Medical College of Georgia. Dr Boesen’s current research interests include control of renal function and blood pressure by the endothelin system, and the role of inflammatory cytokines in hypertension.

Stephen M. Black, PhD, is a Professor in the Vascular Biology Center at the Medical College of Georgia. His education and training include a BSc from the University of Edinburgh, Scotland, UK, and a PhD from the Imperial Cancer Research Fund, UK. After a post-doctoral Fellowship at the University of California, San Francisco, he joined the faculty in the Department of Pediatrics at Northwestern University. He is currently a Professor of Obstetrics & Gynecology and the Basic Science Director of the Cardiovascular Discovery Institute at the Medical College of Georgia. Dr. Black studies the role of oxidative and nitrosative stress in the development of pulmonary vascular disease. His predominant focus is the pulmonary hypertension caused by the increased pulmonary blood flow associated with congenital heart disease.

David M. Pollock, PhD, is a Regents’ Professor of Surgery in the Vascular Biology Center at the Medical College of Georgia. He received his undergraduate degree in biology and chemistry from the University of Evansville, and his doctoral degree from the University of Cincinnati where he studied physiology. He worked as a post-doctoral fellow at the University of North Carolina at Chapel Hill in the Departments of Physiology and Medicine before taking a position in the Drug Discovery Division of Abbott Laboratories 1989. In 1995, he moved to the Medical College of Georgia. He is currently serving as an elected Councilor to the American Physiological Society and Associate Editor of the journals, American Journal of Physiology: Regulatory, Integrative and Comparative Physiology and Vascular Pharmacology. His research interests focus on renal mechanisms of hypertension and the role of endothelin in the control of fluid-electrolyte balance and renal injury. E-mail dpollock{at}mcg.edu; fax 706-721-9799