In Search of Analgesia: Emerging Poles of GPCRs in Pain
- Laura S. Stone1 and
- Derek C. Molliver2
- 1 Faculty of Dentistry, Alan Edwards Centre for Research on Pain, Department of Pharmacology and Therapeutics, McGill University,
Montreal, Quebec, Canada
- 2 Department of Medicine, Pittsburgh Center for Pain Research, University of Pittsburgh, Pittsburgh, PA
Abstract
Of all clinically marketed drugs, greater than thirty percent are modulators of G protein–coupled receptors (GPCRs). Nearly
400 GPCRs (i.e., excluding odorant and light receptors) are encoded within the human genome, but only a small fraction of
these seven-transmembrane proteins have been identified as drug targets. Chronic pain affects more than one-third of the population,
representing a substantial societal burden in use of health care resources and lost productivity. Furthermore, currently available
treatments are often inadequate, underscoring the significant need for better therapeutic strategies. The expansion of the
identified human GPCR repertoire, coupled with recent insights into the function and structure of GPCRs, offers new opportunities
for the development of novel analgesic therapeutics.
Introduction
The G protein–coupled receptors (GPCRs) comprise the largest superfamily of transmembrane receptors. Their function is to
transduce extracellular stimuli into intracellular responses. These stimuli can be remarkably diverse, ranging from physical
stimuli (e.g., photons or heat) to chemical signals in the form of ions (e.g., Ca2+, H+), chemical neurotransmitters (e.g., dopamine, noradrenaline, adrenaline, acetylcholine, or nucleotides), peptides and protein
hormones (e.g., chemokines or opiates), and lipids and eicosanoids (e.g., sphingolipids or leukotrienes). GPCRs mediate and/or
modulate virtually all physiological processes in eukaryotic organisms, including acute and chronic pain (1).
Disorders resulting in persistent pain are among the most common forms of chronic illness in North Americans. In individuals
age sixty and under, the prevalence of migraine and chronic back pain is ten and fifteen percent, respectively. Arthritis
among people less than sixty years of age occurs at a rate of twelve percent, and the frequency rises to forty-six percent
for the population that is older than sixty (2). Medical conditions including diabetes, AIDS, and multiple sclerosis all have a high incidence of chronic neuropathic pain.
Because pain impairs one’s ability to carry out a productive life, it has serious economic consequences in addition to being
a major health problem. In the US alone, an estimated $100 billion is spent each year on health care associated with chronic
pain, and an equal amount is further estimated for the related loss of productivity (3, 4). Available therapeutic interventions, such as morphine, are not always able to adequately control pain; not only is drug
efficacy at issue, but intolerable side effects, such as sedation, respiratory depression, and gastrointestinal impairment,
can also preclude effective pain management. The development of new drugs that target members of the GPCR superfamily holds
great promise for the treatment of acute and chronic pain, reaching far beyond the use of traditional opioid receptor agonists.
In this review, we will first provide an overview of GPCR function with regard to the pain signaling system. Second, we will
discuss emerging insights into GPCR function that relate to nociceptive transmission. Finally, we will conclude with a brief
summary of the role of each GPCR family in nociception.
G Proteins in Signaling
Upon GPCR activation, intracellular signaling systems are activated that couple to a diverse array of downstream effector
systems. By definition, signal transduction through GPCRs involves the heterotrimeric GTP-binding proteins (G proteins) to
which these receptors are coupled. Current estimates, based on the sequencing of the human genome, predict that the G proteins
in human cells can be assembled from among sixteen α, five β, and fourteen γ subunits, with each heterotrimeric combination
corresponding to a distinct complement of effector targets (5, 6). The approximately 400 human GPCRs (exclusive of odorant and light receptors) are differentially expressed by specific tissues,
allowing for a diversity of signaling cascades that may further be localized with respect to distinct intracellular domains
and associated with specific G proteins. In addition, each GPCR may be sensitive to multiple endogenous agonists, and agonists
may act at multiple receptor isoforms. Furthermore, data are emerging that GPCRs also elicit G protein–independent intracellular
effects, further increasing the spectrum of possibilities (7).
The basic cycle of G protein activation and inactivation is illustrated in Figure 1. Agonist binding and receptor activation induce a conformational change in the heterotrimeric G protein such that the α subunit
binds GTP in exchange for GDP, thereby causing the G protein to dissociate into a GTP-bound α monomer and a βγ dimer. The
α monomer and βγ dimer are subsequently free to engage target effectors. A mechanism for terminating G–protein signaling to
effector systems is built in to the α subunit by means of its intrinsic GTPase activity. Hydrolysis of GTP returns the α subunit
to its GDP-bound state, which assembles with the βγ dimer to reform the inactive, heterotrimeric G protein. A number of regulators
of G protein signaling, or RGS proteins, enhance the GTPase function of the α subunit and thereby reduce the duration of GPCR
signaling. The G protein families primarily involved in the modulation of neurotransmission utilize αs, αi/o, or αq/11 subunits (Figure 1B); members within each family show differences in their patterns of expression (8). Downstream effectors also show isoform-specific intracellular targeting and tissue-specific distribution patterns, providing
another level of selectivity in the signaling pathways activated by GPCRs in different cell types.
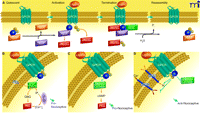
Figure 1
Overview of GPCR signaling. A) Diagram of the cycle of G protein activation and inactivation. In the absence of GPCR signaling (Quiescent), G proteins are
present as inactive αβγ trimers; the α subunit is bound to GDP. Binding of ligand to GPCR (Activation) causes a conformational
change that promotes binding of the receptor to its preferred trimeric G protein and concomitant displacement of bound GDP
by incoming GTP at α subunit. Upon GTP binding, the αβγ trimer dissociates into GTP-bound monomer and βγ dimer, each of which
can then interact with respective effectors. Signaling is terminated by the GTPase activity of the α subunit; this GTPase
activity can be enhanced by RGS proteins. The α subunit–catalyzed hydrolysis of GTP causes subunits to reassemble into the
trimeric G protein. The GPCR is generally desensitized and internalized for recycling or destruction (see text). B–D) Diagrams of the canonical signaling pathways for the major G protein families, as described in the text. (ER, endoplasmic
reticulum.)
Gs proteins (i.e., heterotrimeric G proteins that possess an αs subunit) exert their effects primarily by activating adenylyl cyclase, resulting in increased intracellular cyclic AMP (cAMP),
which in turn activates downstream effectors, including protein kinase A (PKA). Activated PKA phosphorylates numerous proteins
that determine the physiological properties of nociceptors (see below). There are also reports that in some cell types, including
some nociceptors (sensory neurons that detect noxious stimuli), Gs signaling can lead to activation of protein kinase C (PKC) through the cAMP-activated guanine exchange factor Epac (9, 10). These phosphorylation events are regulated in turn by phosphatases and their downstream substrates. PKA may also activate
transcription factors, including the cAMP response element binding protein (CREB), leading to long-term changes in the physiological
properties of affected neurons. Gs activation typically results in increased neuronal excitability.
Gi/o proteins mediate the widespread inhibitory effects of many neurotransmitters. Especially significant for the purposes of
our discussion, Gi/o proteins also mediate the effects of almost all analgesic GPCR agonists. Several mechanisms account for the inhibitory activity
of Gi/o proteins. First, the GTP-bound αi/o subunit inhibits adenyly cyclase, counteracting the effects of Gs activation. Second, the dimer acts to inhibit voltage-dependent calcium channels, resulting in reduced neurotransmitter release
and negative regulation of calcium-activated transcription. Third, they directly hyperpolarize neurons by activation of the
G protein–gated inwardly rectifying potassium channels (GIRKs), which results in reduced excitability. In addition to affecting
channel activity, Gi/o proteins can also modulate neurotransmitter release by interacting directly with release proteins (11). An important function of αi/o subunits is to activate the ERK/MAPK cascade, resulting in regulation of gene expression. [For a comprehensive review of
presynaptic signaling by heterotrimeric G proteins, see (12).]
Gq/11 proteins function mainly through phospholipase C beta (PLCβ), of which there are four known isoforms. PLCβ hydrolyzes membrane
phosphatidylinositol-4,5-bisphosphate (PIP2) to form IP3, which evokes release of intracellular calcium stores (by activation
of IP3 receptors), and diacylglycerol (DAG); both products lead to activation of protein kinase C (PKC). DAG may also activate
protein kinase D. Increased intracellular calcium can promote neurotransmitter release at the presynaptic terminal, activates
calmodulin-dependent mechanisms (e.g., calcium/calmodulin-dependent protein kinase), and may lead to transcription factor
activation. PKC is a major effector for the functional modulation of neuronal signaling machinery downstream of GPCRs. PKCε
appears to be particularly important in the sensitization of primary afferent nociceptors in response to activation of Gq/11 protein–coupled receptors, but other family members contribute to this process as well (13).
Receptor activation can be terminated by G protein–coupled receptor kinases (GRKs) and arrestins [for review, see (14, 15)]. Following prolonged GPCR activation, GRKs phosphorylate the intracellular loops and C terminus of the receptor, which causes
arrestins to associate with the GPCR and promotes receptor internalization. Internalized receptors may be recycled or targeted
for degradation by ubiquitination. For many receptors, desensitization and internalization appear to be separate processes;
the underlying mechanisms are under investigation (14, 15).
In summary, GPCRs alter neuronal functional properties both by covalent modification of the signaling machinery (e.g., phosphorylation)
and transcriptional activation of targeted genes. A basic overview of these pathways is provided in Figure 1. Our knowledge of the diverse pathways activated by GPCRs continues to expand [for review see (16)].
Pain Signaling
Normal nociceptive transmission (see Box 1) begins when nociceptive axons innervating the target organ (e.g., skin, viscera, or joint) are activated by noxious stimuli.
Primary sensory neurons transmit this information from the periphery to the spinal cord dorsal horn, where the nerve impulse
is subject to local modulatory control. A subset of postsynaptic spinal neurons (i.e., secondary sensory neurons) send ascending
axons to the thalamus, where they relay the information to higher cortical centers. The ascending fibers also send collateral
branches into brainstem (i.e., the rostral ventral medulla) and midbrain regions involved in pain modulation (i.e., the periaqueductal
grey) and attention and emotion (i.e., the amygdala). These supraspinal centers in turn send descending projections to the
spinal cord that can either inhibit or facilitate nociception. Many of the analgesic medications currently available target
GPCRs in these descending pathways (17, 18).
Box 1
Pain vs Nociception
Pain is defined by the International Association for the Study of Pain as “an unpleasant sensory and emotional experience
associated with actual or potential tissue damage, or described in terms of such damage.” In contrast, nociception refers
to the transduction of noxious stimuli, irrespective of cognitive awareness. A nociceptor is a sensory neuron preferentially
sensitive to a noxious stimulus or to a stimulus which would become noxious if prolonged. Excitatory and inhibitory influences
on nociception are referred to as pro- or antinociceptive, respectively. Increases and decreases in the experience of pain
are referred to as proalgesic and analgesic, respectively.
In chronic pain conditions, the normal regulation of nociceptive signaling may be altered. For example, inflammatory mediators
released by peripheral tissues and immune cells in response to injury act at GPCRs to sensitize peripheral nociceptors (see
Box 1), making them more responsive to both noxious and innocuous stimuli. Persistent firing of peripheral nociceptors causes spinal
cord neurons to become more responsive to nociceptive input through a process known as central sensitization. Sensitization
also occurs at higher-order relays in the brain. Neurons at each step in the pain pathway, both ascending and descending,
are subject to modulation by GPCRs that thus represent potential targets for therapeutic intervention into persistent pain.
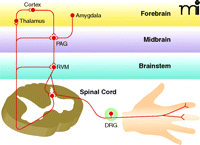
An overview of the pain signaling system is provided in Figure 2.
Figure 2
Overview of pain transmission. Nociception begins in the periphery with the activation of nociceptive sensory neurons by noxious stimuli (e.g., heat, acid,
or tissue injury). These neurons, which have their cell bodies in the dorsal root ganglia (DRG), synapse on neurons in the
spinal cord that send ascending projections to the thalamus, which in turn projects to forebrain regions involved in the subjective
experience of pain. Descending inhibitory and excitatory pathways are activated by both ascending input from the spinal cord
and descending input from the forebrain and limbic structures, including the amygdala. The major structures modulating descending
modulation are found in the brainstem rostral ventral medulla (RVM) and midbrain regions [periaqueductal grey, PAG)].
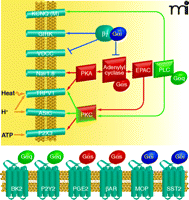
Figure 3
GPCR modulation of nociceptor excitability. GPCR activation, in response to tissue damage or inflammatory mediators, can often result in the covalent modification (e.g.,
phosphorylation) of ion channels; such channel modification can modulate important physiological properties of nociceptors.
A few examples of GPCRs and ion channels that are involved in the modulation of nociceptor activity are shown (see text for
details); many other GPCRs have also been implicated in this process. GPCRs also regulate functional properties of neurons
at the level of transcription (not shown here). Channels that are regulated by GPCR activation include the ligand-gated ion
channels TRPV1, ASICs, and P2X3; voltage-dependent channels including tetrodotoxin-resistant sodium channels Nav1.8 and Nav1.9;
voltage-dependent calcium channels; KCNQ channels mediating the M-type potassium current; and G protein–activated potassium
channels (GIRK). GPCR-mediated pathways regulating channel function represent an active area of investigation.
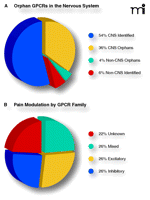
Figure 4
Current status and opportunities in GPCR targeting. A) Predicted proportions of human GPCRs by nervous system expression (90%) and orphan status (40%). Approximately one-third
of all GPCR genes encode orphan receptors that are expressed in the CNS. The endogenous ligand(s) and physiological function(s)
of these receptors remain to be discovered, representing enormous opportunities for drug development. B) Modulatory effects of GPCRs organized by family. The equal distribution of inhibitory, excitatory, and mixed functional families
suggests an equal balance between pro- and anti-nociception.
Emerging Concepts in GPCR Signaling and Pain Modulation
GPCR Signaling and Modulation of Peripheral Nociceptive Channels
GPCRs modulate the function of a wide variety of ion channels and signaling molecules in sensory neurons, allowing neurons
to rapidly adjust their sensitivity in response to changes in peripheral target tissues and at the central synapse. In particular,
GPCRs modulate ligand-gated and voltage-dependent ion channels that determine key physiological characteristics of nociceptors
(19). These channels include members of the transient receptor potential (TRP) family of ligand-gated cation channels, such as
TRPV1 and TRPA1, ATP-gated P2X channels, acid-sensing ion channels (ASICs), TTX-resistant sodium channels, voltage-dependent
calcium channels, and M-type potassium channels.
TRPV1, a cation channel gated by heat and protons, is selectively expressed in a subset of primary afferent nociceptors and
plays a key role in the sensitization of nociceptors in response to inflammation. Numerous GPCRs have been found to regulate
TRPV1 (20, 21). Both Gs and Gq protein–coupled receptor signaling enhance TRPV1 function, resulting in peripheral sensitization of nociceptors and reduced
pain threshold. Activation of PKCε by Gq signaling plays a major role in the modulation of TRPV1. A related family member, TRPA1, shares many of the same regulatory
mechanisms and is largely co-expressed with TRPV1. Several studies suggest that constitutive modulation of TRPV1 by GPCR signaling
is required to maintain normal TRPV1 function (20). Additional TRP family members have also been implicated in the transduction of thermal stimuli (21).
P2X3 is a member of the P2X family of ATP-gated ion channels that is preferentially expressed in the non-peptidergic subset
of nociceptive sensory neurons. During inflammation, P2X3 currents are enhanced through phosphorylation by PKC. This occurs
through an indirect pathway in which the Gs protein–coupled prostaglandin receptor PGE2 acts through Epac1 to activate PKC (9). ASICs are also positively regulated by GPCRs, including serotonin receptors, through the action of PKC. Phosphorylation
appears to be selective for the ASIC2b subunit, although it affects currents through heteromeric channels, including those
that contain ASIC3 subunits (22). Whereas ASIC2b is expressed by many sensory neurons, ASIC3 is more restricted to peptidergic nociceptors and a subset of
larger-diameter neurons of unknown modality (23).
M-type potassium currents, generated by channels consisting of KCNQ subunits, play a key role in regulating nociceptor sensitivity.
M-type potassium currents are negatively modulated through Gq protein–mediated signaling; this negative modulation acts to depolarize the resting membrane potential and thereby enhances
nociceptive signaling (24). Voltage-dependent calcium channels regulate action potential kinetics and neurotransmitter release as well as neuronal activity–dependent
transcription. In nociceptors, these channels contribute significantly to the duration of the action potential. Gi/o protein–coupled receptors such as the opioid receptors inhibit primary afferent signaling in part by inhibiting calcium channels
through a direct action of the G protein βγ subunits (25).
Tetrodotoxin-resistant sodium channels Nav1.8 and Nav1.9 are selectively expressed in nociceptors and contribute to injury-evoked
changes in neuronal excitability. These channels are modulated through GPCR signaling cascades initiated by inflammatory mediators
such as prostaglandins, serotonin, and adenosine (19). Regulation of these sodium channels can occur both through Gs signaling through PKA, which is antagonized by Gi, and by Gq activation of PKC.
Protein Scaffolding and the Organization of Signaling Molecules
The great diversity of GPCR signaling entails a large number of receptors that must evoke selective, often tissue-specific,
cellular responses, despite a relatively small complement of G proteins. There is increasing evidence that the functional
selectivity of GPCRs is tightly regulated through targeting of signaling components to macromolecular signaling complexes
(so-called trans-ducisomes) at specialized membrane compartments known as lipid rafts. Lipid rafts are membrane domains of
reduced fluidity, enriched in cholesterol and glycosphingolipids, that promote the assembly of signaling protein complexes
(26). These complexes are organized through protein–protein interaction domains (e.g., PDZ, SH2, and SH3 domains) and specialized
scaffold proteins that physically coordinate the signaling effector molecules in the transducisome (27). This structural organization allows for highly-efficient regulation of effector function. For example, in some cells, Gq protein–coupled glutamate receptors in the plasma membrane are physically associated with the IP3 receptors on endoplasmic
reticulum that regulate calcium stores. This process occurs through binding of the scaffolding protein Homer (28). In nociceptors, some ion channels modulated by G protein signaling are also associated with the transducisome. Examples of
channels that are rapidly modulated upon nociceptive GPCR activation and are likely to be associated with scaffolding proteins
include the heat- and acid-gated channel TRPV1 and the M-type potassium channel (discussed above) (29).
Available evidence suggests that GPCRs and G proteins are modified by fatty acid acylation, particularly palmitoylation and
myristylation, and that these modifications, along with specific polypeptide sequences within the GPCR transmembrane domains,
are responsible for directing these proteins to lipid rafts. However, other mechanisms, as yet unidentified, are likely to
be important in determining whether a GPCR is targeted to lipid rafts (30). The formation of signaling complexes in lipid rafts provides a mechanism for specialized and highly efficient signal transduction,
with pathway selectivity determined by the association of specific effector molecules and receptors through scaffolding proteins.
Integrins are transmembrane proteins associated with lipid rafts and mediate focal adhesions at which the intracellular cytoskeleton
connects to extracellular matrix. Integrins contribute to the formation of signaling complexes that are activated in response
to binding of extracellular matrix proteins. Studies by Levine and colleagues indicate that integrin binding to the extra-cellular
matrix, along with intact lipid rafts, is essential for signaling through a number of GPCRs in inflammatory hyperalgesia (31). The extent to which aberrant nociceptive GPCR signaling or malformation of signaling complexes might underlie persistent
pain states is a largely untapped area of investigation.
Ligand-and G Protein–Independent GPCR Signaling
There have been several reports that GPCRs can engage with components of the intracellular signaling complex and activate
G protein signaling in the absence of extracellular ligands. Signaling complex components that have been implicated in such
interactions include adhesion molecules such as integrins, scaffolding molecules such as Homer, and growth factor receptor
tyrosine kinases such as the nerve growth factor receptor TrkA and the epidermal growth factor receptor. This kind of receptor
transactivation has been described in both directions; GPCRs may also activate signaling through receptor tyrosine kinases
or integrins (16).
In addition, increasing evidence supports the idea that signaling by GPCRs may occur independently of G proteins. The molecules
most clearly involved in this process are the β-arrestins, which are widely understood to be involved in the desensitization
and recycling of GPCRs. β-arrestins are also able to function as scaffolding molecules for GPCRs and downstream effectors,
such as Src tyrosine kinase family members and the MAP kinases, and may actually allow transactivation of these molecules
independently of G protein actions (32). However, pathways also exist for the activation of MAP kinases by G protein subunits, suggesting that the pathway used for
activation of a specific effector molecule in a given cell type is highly context-dependent (16). These data raise the possibility that there are GPCR-mediated effects in nociceptors that are ligand- and/or G protein–independent
(33).
GPCR Oligomerization
It is now recognized that GPCRs, traditionally envisaged monomeric, can form oligomeric complexes. These associations can
result in novel pharmacological properties distinct from either component receptor, including alterations in ligand binding
affinity, changes in signal transduction, and altered receptor trafficking [for review see (34–37)]. The recognition of oligomeric GCPRs has led to significant re-evaluation of the in vivo mechanisms thought to be involved
in GPCR function. Homo- and hetero-oligomerization has been documented within GPCR families, (e.g., the opioid receptor family)
and across GPCR families. For example, the functional implications of GPCR–GPCR interactions includes the “unmasking” of opioid
binding sites when both the μ- and δ-opioid receptors are co-expressed (38). In addition, the formation of functional GABAB receptors is predicated on a requirement for co-expression of both GABABR1/GABABR2 receptor species (39–41). Although the potential in vivo relevance of these data for neuronal function remains an open question, the existence of
oligomers has significant implications for drug development. For example, if the functional receptor is heteromeric, strategies
to identify GPCR ligands that rely on cell systems expressing only a single receptor type may not be successful. It is therefore
possible that the large number of currently orphaned GPCRs reflects the use of screening paradigms that rely on monomeric
rather than heteromeric systems.
Regulation of Cell Surface Expression
The regulation of GPCR internalization and recycling to the cell surface following agonist activation is an area of intense
research and has been reviewed extensively elsewhere (42, 43). An interesting new development, with relevance to analgesic drug discovery, is the observation that receptor signaling,
internalization, desensitization, and recycling can differ, depending on the specific ligand used (44). This ligand-specific regulation has enormous implications for the development of clinically useful agents with reduced risk
of tolerance.
To become functionally competent, GPCRs must be properly synthesized and trafficked to the cell membrane, processes that are
under tight cellular control [for review, see (7)]. The cell surface expression of the δ-opioid receptor (DOP) subtype is a case in point. In axon terminals, DOP is associated
with large, dense-core vesicles (LDCVs) and in sensory and spinal cord neuron cell bodies, expression is primarily intracellular.
In both axons and cell bodies, DOP appears to be inserted into the plasma membrane in a stimulus-dependent manner [see (37, 45, 46)]. DOP may also be translocated in response to chronic morphine exposure, peripheral inflammation, inflammatory mediators,
and chronic nociceptive stimuli. As a consequence, sensitivity to DOP agonists is increased. For example, chronic morphine
treatment results in an increase both in intrathecal DOP agonist–induced analgesia and in the number of plasma membrane–associated
DOP-immunoreactive particles (47).
GPCR Families in Pain Modulation
Early attempts to study, classify, and target GPCRs relied on measurable functional endpoints and on the availability of compounds
to selectively stimulate or antagonize those responses. Historically, the modification of these compounds provided the primary
approach to the development of new drugs with improved properties. For example, there are at least two dozen different chemical
entities in clinical use that target opioid receptors (e.g., morphine and methadone), and most exist in multiple formulations
optimized with regard for route of administration or half-life in the plasma. As a result, currently available drugs target
only a small fraction of GPCRs.
The human genome project has identified more than 800 different GPCRs, approximately half of which are predicted to respond
to endogenous (non-light and non-odorant) ligands (48, 49). Of the 379 GPCRs (i.e., exclusive of odorant and light receptors) by the International Union of Basic and Clinical Pharmacology
Committee on Receptor Nomenclature and Drug Classification (NC-IUPHAR), an estimated ninety percent are expressed in the central
nervous system (50), and nearly forty percent of all GPCRs remain orphans with no identified ligand (49). These numbers predict that over 100 new GPCRs of currently unknown function remain to be identified in the central nervous
system, and indeed, new potential targets for drug development have been identified along with previously unknown neurotransmitters.
Further insights into GPCR identification and characterization will undoubtedly advance our understanding of pain transmission.
We used the NC-IUPHAR classification system (www.iuphar.org/nciuphar.html) to survey the role(s) of each of the currently proposed GPCR families in pain processing. We were astounded to discover
that nearly eighty percent (47/61) of the currently identified families have a known role in the modulation of pain. These
survey results speak to the enormous physiological importance of pain modulation by GPCRs. A brief summary of our current
understanding of the role of each GPCR family in nociception is provided in Table 1.
Table 1
GPCR Families and Their G Protein–Dependent Roles in Pro- and Antinociceptive Processing
Conclusions
Regulation of pain transmission by GPCRs occurs throughout the central nervous system, providing a dominant focus for clinical
analgesic therapy. However, modulation of nociceptive transduction and processing also occurs in the primary afferent neurons
and in peripheral tissues, and all of these sites represent potential targets for novel analgesics. Indeed, it is becoming
increasingly clear that GPCRs provide a fundamental mechanism of regulation in an integrated network of communication among
sensory axon terminals, their peripheral target tissues, and immune cells (20). The identification of receptors and mechanisms of regulation of GPCRs in pain transmission remains a fertile and largely
unexplored field for the development of novel therapeutics for acute and chronic pain, particularly given the paucity of currently
available drugs.

Acknowledgments
Acknowledgments
LSS is supported by CIHR MOP-86691 and FRSQ Bourse de chercheur-boursier. DCM is supported by the National Institutes of Health
[Grant NS056122].
References
- ↵
Römpler, H, Stäubert, C, Thor, D, Schulz, A, Hofreiter, M and Schöneberg, T. G protein–coupled time travel: Evolutionary aspects of
GPCR research. Mol Interv7, 17–25 (2007).
- ↵
Rapoport, J, Jacobs, P, Bell, NR and Klarenbach, S. Refining the measurement of the economic burden of chronic diseases in Canada. Chronic Dis Can 25, 13–21 (2004.).
- ↵
McCarberg, BH and Billington, R. Consequences of neuropathic pain: Quality-of-life issues and associated costs. Am J Manag Care 12, S263–268 (2006).
- ↵
Stewart, WF, Ricci, JA, Chee, E, Morganstein, D and Lipton, R. Lost productive time and cost due to common pain conditions in the US
work-force. JAMA 290, 2443–2454 (2003).
- ↵
Milligan, G and Kostenis, E. Heterotrimeric G proteins: A short history. Br J Pharmacol 147, S46–S55 (2006).
- ↵
Sanders, RD, Brian, D and Maze, M. G protein–coupled receptors. Handb Exp Pharmacol 182, 93–117 (2008).
- ↵
Dupre, DJ and Hebert, TE. Biosynthesis and trafficking of seven-trans-membrane receptor signalling complexes. Cell Signal 18, 1549–1559 (2006).
- ↵
Offermanns, S. G proteins as transducers in transmembrane signalling. Prog Biophys Mol Biol83, 101–130 (2003).
- ↵
Wang, C, Gu, Y, Li, GW and Huang, LY. A critical role of the cAMP sensor Epac in switching protein kinase signalling in prostaglandin
E2-induced potentiation of P2X3 receptor currents in inflamed rats. J Physiol584, 191–203 (2007). This study demonstrated that the pro-inflammatory mediator prostaglandin E2, which activates G protein–coupled prostanoid
receptors, produces a large increase in ATP-gated currents. This is an example of GPCR-mediated modulation of ion channel
function in sensory neurons and suggests a mechanism contributing to inflammation-induced peripheral sensitization..
- ↵
Hucho, TB, Dina, OA and Levine, JD. Epac mediates a cAMP-to-PKC signaling in inflammatory pain: An isolectin B4(+) neuron-specific
mechanism. J Neurosci25, 6119–6126 (2005).
- ↵
Kupchik, YM, Rashkovan, G, Ohana, L, Keren-Raifman, T, Dascal, N, Parnas, H and Parnas, I. Molecular mechanisms that control initiation
and termination of physiological depolarization-evoked transmitter release. Proc Natl Acad Sci USA105, 4435–4440 (2008).
- ↵
Brown, DA and Sihra, TS. Presynaptic signaling by heterotrimeric G proteins. Handb Exp Pharmacol 18, 207–260 (2008).
- ↵
Khasar, SG, Lin, YH, Marti, A, et al. A novel nociceptor signaling pathway revealed in protein kinase C epsilon mutant mice. Neuron24, 253–260 (1999).
- ↵
Tilakaratne, N and Sexton, PM. G protein–coupled receptor–protein interactions: Basis for new concepts on receptor structure and function.
Clin Exp Pharmacol Physiol 32, 979–987 (2005).
- ↵
Gainetdinov, RR, Premont, RT, Bohn, LM, Lefkowitz, RJ and Caron, MG. Desensitization of G protein–coupled receptors and neuronal functions.
Annu Rev Neurosci27, 107–144 (2004).
- ↵
Rozengurt, E. Mitogenic signaling pathways induced by G protein–coupled receptors. J Cell Physiol213, 589–602 (2007).
- ↵
Millan, MJ. Descending control of pain. Prog Neurobiol 66, 355–474 (2002).
- ↵
Pan, HL, Wu, ZZ, Zhou, HY, Chen, SR, Zhang, HM and Li, DP. Modulation of pain transmission by G protein–coupled receptors. Pharmacol Ther117, 141–161 (2008).
- ↵
McCleskey, EW and Gold, MS. Ion channels of nociception. Annu Rev Physiol 61, 835–856 (1999).
- ↵
Dussor, G, Koerber, HR, Oaklander, AL, Rice, FL and Molliver, DC. Nucleotide signaling and cutaneous mechanisms of pain transduction.
Brain Res Rev60, 24–35 (2009).
- ↵
Patapoutian, A, Tate, S and Woolf, CJ. Transient receptor potential channels: Targeting pain at the source. Nat Rev Drug Discov8, 55–68 (2009).
- ↵
Deval, E, Salinas, M, Baron, A, Lingueglia, E and Lazdunski, M. ASIC2b-dependent regulation of ASIC3, an essential acid-sensing ion
channel subunit in sensory neurons via the partner protein PICK-1. J Biol Chem279, 19531–19539 (2004).
- ↵
Molliver, DC, Immke, DC, Fierro, L, Paré, M, Rice, FL and McCleskey, EW. ASIC3, an acid-sensing ion channel, is expressed in metaboreceptive
sensory neurons. Mol Pain1, 35 (2005).
- ↵
Passmore, GM, Selyenko, AA, Mistri, M, et al. KCNQ/M currents in sensory neurons: significance for pain therapy. J Neurosci23, 7227–7236 (2003).
- ↵
Strock, J and Diverse-Pierluissi, MA. Ca2+ channels as integrators of G protein-mediated signaling in neurons. Mol Pharmacol 66, 1071–1076 (2004).
- ↵
Insel, PA, Head, BP, Patel, HH, Roth, DM, Bundey, RA and Swaney, JS. Compartmentation of G protein–coupled receptors and their signalling
components in lipid rafts and caveolae. Biochem Soc Trans33, 1131–1134 (2005).
- ↵
Pawson, T and Scott, JD. Signaling through scaffold, anchoring, and adaptor proteins. Science 278, 2075–2080 (1997).
- ↵
Hoshi, N, Langeberg, LK and Scott, JD. Distinct enzyme combinations in AKAP signalling complexes permit functional diversity. Nat Cell Biol7, 1066–1073 (2005).
- ↵
Qanbar, R and Bouvier, M. Role of palmitoylation/depalmitoylation reactions in G protein–coupled receptor function. Pharmacol Ther 97, 1–33 (2003).
- ↵
Dina, O, Hucho, T, Yeh, J, Malik-Hall, M, Reichling, D and Levine, J. Primary afferent second messenger cascades interact with specific
inte-grin subunits in producing inflammatory hyperalgesia. Pain115, 191–203 (2005). This study was among the first to suggest that integrins play a critical role in inflammatory pain by interacting with
components of second messenger cascades that mediate inflammatory hyperalgesia, and that such interactions may be organized
by lipid rafts..
- ↵
Delcourt, N, Bockaert, J and Marin, P. GPCR-jacking: From a new route in RTK signalling to a new concept in GPCR activation. Trends Pharmacol Sci28, 602–607 (2007).
- ↵
Hall, RA, Ostedgaard, LS, Premont, RT, Blitzer, JT, Rahman, N, Welsh, MJ and Lefkowitz, RJ. A C-terminal motif found in the beta2-adrenergic
receptor, P2Y1 receptor and cystic fibrosis transmembrane conductance regulator determines binding to the Na+/H+ exchanger
regulatory factor family of PDZ proteins. Proc Natl Acad Sci USA95, 8496–8501 (1998).
- ↵
de Bartolomeis, A and Iasevoli, F. The Homer family and the signal transduction system at glutamatergic postsynaptic density: Potential role
in behavior and pharmacotherapy. Psychopharmacol Bull 37, 51–83 (2003).
- ↵
George, SR, O’Dowd, BF and Lee, SP. G protein–coupled receptor oligomerization and its potential for drug discovery. Nat Rev Drug Discov1, 808–820 (2002).
-
Bulenger, S, Marullo, S and Bouvier, M. Emerging role of homo- and heterodimerization in G protein–coupled receptor biosynthesis and
maturation. Trends Pharmacol Sci26, 131–137 (2005).
-
Prinster, SC, Hague, C and Hall, RA. Heterodimerization of G protein coupled–receptors: Specificity and functional significance. Pharmacol Rev57, 289–298 (2005).
- ↵
Milligan, G. G protein-coupled receptor hetero-dimerization: Contribution to pharmacology and function. Br J Pharmacol158, 1–4 (2009).
- ↵
Gomes, I, Jordan, BA, Gupta, A, Trapaidze, N, Nagy, V and Devi, LA. Heterodimerization of mu and delta opioid receptors: A role in
opiate synergy. J Neurosci20, RC110 (2000).
- ↵
Kaupmann, K, Malitschek, B, Schuler, V, et al. GABA(B)-receptor subtypes assemble into functional heteromeric complexes. Nature396, 683–687 (1998).
-
White, JH, Wise, A, Main, MJ, et al. Heterodimerization is required for the formation of a functional GABA(B) receptor. Nature396, 679–682 (1998).
- ↵
Jones, KA, Borowsky, B, Tamm, JA, et al. GABA(B) receptors function as a heteromeric assembly of the subunits GABA(B)R1 and GABA(B)R2.
Nature396, 674–679 (1998).
- ↵
Drake, MT, Shenoy, SK and Lefkowitz, RJ. Trafficking of G protein–coupled receptors. Circ Res99, 570–582 (2006).
- ↵
Moore, CA, Milano, SK and Benovic, JL. Regulation of receptor trafficking by GRKs and arrestins. Annu Rev Physiol69, 451–482 (2007).
- ↵
Pineyro, G and Archer-Lahlou, E. Ligand-specific receptor states: implications for opiate receptor signalling and regulation. Cell Signal 19, 8–19 (2007).
- ↵
Zhang, X, Bao, L and Guan, JS. Role of delivery and trafficking of delta-opioid peptide receptors in opioid analgesia and tolerance.
Trends Pharmacol Sci27, 324–329 (2006).
- ↵
Cahill, CM, Holdridge, SV and Morinville, A. Trafficking of delta-opioid receptors and other G protein–coupled receptors: Implications
for pain and analgesia. Trends Pharmacol Sci28, 23–31 (2007).
- ↵
Cahill, CM, Morinville, A, Lee, MC, Vincent, JP, Collier, B and Beaudet, A. Prolonged morphine treatment targets delta opioid receptors
to neuronal plasma membranes and enhances delta-mediated antinociception. J Neurosci21, 7598–7607 (2001). This study demonstrated an increase in targeting of the delta opioid receptor (DOP)-immunoreactive particles to the plasma
membranes of spinal cord neurons following chronic morphine exposure in the absence of neosynthesis. This translocation was
associated with a marked potentiation in DOP-mediated spinal antinociception, suggesting that DOP targeting to the plasma
membrane may be a mechanism by which opioid receptor activation is regulated in vivo. The regulation of cell surface expression
is emerging as an important regulatory mechanism for some GPCRs..
- ↵
Foord, SM, Bonner, TI, Neubig, RR, Rosser, EM, Pin, JP, Davenport, AP, Spedding, M and Harmar, AJ. International Union of Pharmacology.
XLVI. G protein–coupled receptor list. Pharmacol Rev57, 279–288 (2005).
- ↵
Harmar, AJ, Hills, RA, Rosser, EM, et al. IUPHAR-DB: The IUPHAR database of G protein–coupled receptors and ion channels. Nucleic Acids Res37, D680–D685 (2009).
- ↵
Vassilatis, DK, Hohmann, JG, Zeng, H, et al. The G protein–coupled receptor repertoires of human and mouse. Proc Natl Acad Sci USA100, 4903–4908 (2003). This study used bioinformatics to describe a total of 367 GPCRs for endogenous ligands in the human genome, including previously
unidentified receptors. Expression profiling of 100 of the identified GPCRs demonstrated that the majority are expressed in
more than one tissue and over 90% are expressed in the brain. Sequence analysis was used to predict the ligand type for dozens
of orphan receptors. This study illustrates the vast untapped potential of uncharacterized GPCRs in neurobiology..
- ↵
Sommer, C. Serotonin in pain and analgesia: Actions in the periphery. Mol Neurobiol30, 117–125 (2004).
- ↵
Eisenach, JC. Muscarinic-mediated analgesia. Life Sci64, 549–554 (1999).
- ↵
Wess, J, Duttaroy, A, Gomeza, J, et al. Muscarinic receptor subtypes mediating central and peripheral antinociception studied with
muscarinic receptor knockout mice: A review. Life Sci72, 2047–2054 (2003).
- ↵
Sawynok, J. Adenosine receptor activation and nociception. Eur J Pharmacol347, 1–11 (1998).
-
Gomes, JA, Li, X, Pan, HL and Eisenbach, JC. Intrathecal adenosine interacts with a spinal noradrenergic system to produce antinociception
in nerve-injured rats. Anesthesiology91, 1072–1079 (1999).
- ↵
Zylka, MJ, Sowa, NA, Taylor-Blake, B, Twomey, MA, Herrala, A, Voikar, V and Vihko, P. Prostatic acid phosphatase is an ectonucleoti-dase
and suppresses pain by generating adenosine. Neuron60, 111–122 (2008). This study demonstrated that a previously unidentified extracellular enzyme localized at nociceptor terminals in the spinal
cord represents a mechanism for endogenous analgesia by producing adenosine to act at anti-nociceptive adenosine receptors.
The authors devised a novel approach for exploiting this endogenous mechanism by intrathecally injecting soluble enzyme to
produce powerful analgesia in chronic pain models..
- ↵
Teasell, RW and Arnold, JM. Alpha-1 adrenoceptor hyperresponsive-ness in three neuropathic pain states: complex regional pain syndrome
1, diabetic peripheral neuropathic pain and central pain states following spinal cord injury. Pain Res Manag 9, 89–97 (2004).
-
Fairbanks, CA, Stone, LS and Wilcox, GL. Pharmacological profiles of alpha 2 adrenergic receptor agonists identified using genetically
altered mice and isobolographic analysis. Pharmacol Ther123, 224–238 (2009).
-
Deyama, S, Katayama, T, Ohno, A, Nakagawa, T, Kaneko, S, Yamaguchi, T, Yoshioka, M and Minami, M. Activation of the beta-adrenoceptor-protein
kinase A signaling pathway within the ventral bed nucleus of the stria terminalis mediates the negative affective component
of pain in rats. J Neurosci28, 7728–7736 (2008).
- ↵
Nackley, AG, Tan, KS, Fecho, K, Flood, P, Diatchenko, L and Maixner, W. Catechol-O-methyltransferase inhibition increases pain sensitivity
through activation of both beta2- and beta3-adrenergic receptors. Pain128, 199–208 (2007).
- ↵
Takai, S, Song, K, Tanaka, T, Okunishi, H and Miyazaki, M. Antinoci-ceptive effects of angiotensin-converting enzyme inhibitors and
an angio-tensin II receptor antagonist in mice. Life Sci59, PL331–PL336 (1996).
- ↵
Kaneko, S, Mori, A, Tamura, S, Satoh, M and Takagi, H. Intracerebro-ventricular administration of angiotensin II attenuates morphine-induced
analgesia in mice. Neuropharmacology24, 1131–1134 (1985).
- ↵
Twining, CM, et al. Activation of the spinal cord complement cascade might contribute to mechanical allodynia induced by three animal
models of spinal sensitization. J Pain6, 174–183 (2005).
-
Clark, JD, Oiao, Y, Li, X, Shi, X, Angst, MS and Yeomans, DC. Blockade of the complement C5a receptor reduces incisional allodynia,
edema, and cytokine expression. Anesthesiology104, 1274–1282 (2006).
-
Griffin, RS, Costigan, M, Brenner, GJ, et al. Complement induction in spinal cord microglia results in anaphylatoxin C5a-mediated pain
hypersensitivity. J Neurosci27, 8699–8708 (2007). This study identified several immune-related components that were highly regulated in spinal cords following peripheral
nerve injury including complement component C5 and the C5a receptor (C5aR), which were upregulated in spinal microglia after
peripheral nerve injury. Furthermore, intrathecal administration of C5a produced hypersensitivity in naive mice and a C5aR
antagonist reduced behavioral signs of neuropathic pain. This study illustrates several important concepts, including the
potential importance of non-neuronal targets, the emerging role of neuroimmune interactions in chronic pain, and the ability
of new technologies to reveal previously unanticipated roles for GPCRs in pain and analgesia..
- ↵
Ting, E, Guerrero, AT, Cunha, TM, et al. Role of complement C5a in mechanical inflammatory hypernociception: Potential use of C5a
receptor antagonists to control inflammatory pain. Br J Pharmacol153, 1043–1053 (2008).
- ↵
Xu, N, Wang, H, Fan, L and Chen, O. Supraspinal administration of apelin-13 induces antinociception via the opioid receptor in
mice. Peptides30, 1153–1157 (2009).
- ↵
Zhang, L, Zhang, X and Westlund, KN. Restoration of spontaneous exploratory behaviors with an intrathecal NMDA receptor antagonist
or a PKC inhibitor in rats with acute pancreatitis. Pharmacol Biochem Behav77, 145–153 (2004).
- ↵
Traub, RJ, Tang, B, Ji, Y, Pandya, S, Yfantis, H and Sun, Y. A rat model of chronic postinflammatory visceral pain induced by deoxycholic
acid. Gastroenterology135, 2075–2083 (2008).
- ↵
Pert, A, Moody, TW, Pert, CB, Dewald, LA and Rivier, J. Bombesin: Receptor distribution in brain and effects on nociception and locomotor
activity. Brain Res193, 209–220 (1980).
- ↵
Cridland, RA and Henry, JL. Bombesin, neuromedin C and neuromedin B given intrathecally facilitate the tail flick reflex in the rat. Brain Res 584, 163–168 (1992).
- ↵
Vellani, V, Zachrisson, O and McNaughton, PA. Functional bradykinin B1 receptors are expressed in nociceptive neurones and are upregulated
by the neurotrophin GDNF. J Physiol560, 391–401 (2004).
-
Wang, H, Kono, T, Amaya, F, et al. Bradykinin produces pain hypersensitivity by potentiating spinal cord glutamatergic synaptic transmission.
J Neurosci25, 7986–7992 (2005).
- ↵
Prado, GN, et al. Mechanisms regulating the expression, self-maintenance, and signaling-function of the bradykinin B2 and B1 receptors.
J Cell Physiol193, 275–286 (2002).
- ↵
Ma, W, Chabot, JG and Quirion, R. A role for adrenomedullin as a pain-related peptide in the rat. Proc Natl Acad Sci USA103, 16027–16032 (2006).
-
Tepper, SJ and Stillman, MJ. Clinical and preclinical rationale for CGRP-receptor antagonists in the treatment of migraine. Headache 48, 1259–1268 (2008).
- ↵
Gennari, C. Analgesic effect of calcitonin in osteoporosis. Bone30, 67S–70S (2002).
- ↵
Hosking, RD and Zajicek, JP. Therapeutic potential of cannabis in pain medicine. Br J Anaesth 101, 59–68 (2008).
- ↵
Malan, TP, Jr, Ibrahim, MM, Lai, J, Vanderah, TW, Makrivannis, A and Porreca, F. CB2 cannabinoid receptor agonists: Pain relief without
psychoactive effects. Curr Opin Pharmacol3, 62–67 (2003).
- ↵
White, FA, Jung, H and Miller, RJ. Chemokines and the pathophysiol-ogy of neuropathic pain. Proc Natl Acad Sci USA104, 20151–20158 (2007).
- ↵
Marchand, F, Perretti, M and McMahon, SB. Role of the immune system in chronic pain. Nat Rev Neurosci6, 521–532 (2005).
-
Scholz, J and Woolf, CJ. The neuropathic pain triad: Neurons, immune cells and glia. Nat Neurosci 10, 1361–1368 (2007).
- ↵
Abbadie, C. Chemokines, chemokine receptors and pain. Trends Immunol26, 529–534 (2005).
- ↵
Xie, JY, Herman, DS, Stiller, CO, et al. Cholecystokinin in the rostral ventromedial medulla mediates opioid-induced hyperalgesia
and anti-nociceptive tolerance. J Neurosci25, 409–416 (2005).
- ↵
Gallagher, JP, Orozco-Cabal, LF, Liu, J and Shinnick-Gallagher, P. Synaptic physiology of central CRH system. Eur J Pharmacol583, 215–225 (2008).
- ↵
Bomholt, SF, Harbuz, MS, Blackhorn-Monro, G and Blackhorn-Monro, RE. Involvement and role of the hypothalamo-pituitary-adrenal (HPA)
stress axis in animal models of chronic pain and inflammation. Stress7, 1–14 (2004).
- ↵
McFarlane, AC. Stress-related musculoskeletal pain. Best Pract Res Clin Rheumatol21, 549–565 (2007).
- ↵
Potvin, S, Grignon, S and Marchand, S. Human evidence of a supra-spinal modulating role of dopamine on pain perception. Synapse63, 390–402 (2009).
- ↵
Khodorova, A, Navarro, B, Jouaville, LS, et al. Endothelin-B receptor activation triggers an endogenous analgesic cascade at sites of
peripheral injury. Nat Med9, 1055–1061 (2003).
- ↵
Khodorova, A, Fareed, MU, Gokin, A, Strichartz, GR and Davar, G. Local injection of a selective endothelin-B receptor agonist inhibits
endothelin-1-induced pain-like behavior and excitation of nociceptors in a naloxone-sensitive manner. J Neurosci22, 7788–7796 (2002).
- ↵
Liverman, CS, Brown, JW, Sandhir, R, McCarson, KE and Berman, NE. Role of the oestrogen receptors GPR30 and ERalpha in peripheral sensitization:
Relevance to trigeminal pain disorders in women. Cephalalgia29, 729–741 (2009). This study reports a pronocicep-tive role for the estrogen receptor GPER (formerly known as the orphan receptor GPR30)
in inflammation-induced sensitization. Although the modulatory effects of estrogen on nociception are well described, they
had previously been attributed exclusively to the nuclear steroid hormone receptors ERα and ERβ. The discovery of GPER may
lead to significant revision of our understanding of estrogen-mediated modulation of nociception. GPER is an excellent example
of how GPCR genomics is expected to revolutionize our understanding of CNS modulatory processes..
- ↵
Becker, EL, Forouhar, FA, Grunnet, ML, et al. Broad immunocy-tochemical localization of the formylpeptide receptor in human organs,
tissues, and cells. Cell Tissue Res292, 129–135 (1998).
- ↵
Egger-Adam, D and Katanaev, VL. Trimeric G protein–dependent signaling by Frizzled receptors in animal development. Front Biosci 13, 4740–4755 (2008).
- ↵
Luyten, FP, Tylzanowski, P and Lories, RJ. Wnt signaling and osteoarthritis. Bone44, 522–527 (2009).
- ↵
Xu, XJ, Hokfelt, T and Wiesenfeld-Hallin, Z. Galanin and spinal pain mechanisms: Where do we stand in 2008. Cell Mol Life Sci65, 1813–1819 (2008).
- ↵
Lundstrom, L, Elmquist, A, Bartfai, T and Langel, U. Galanin and its receptors in neurological disorders. Neuromolecular Med7, 157–180 (2005).
- ↵
Sibilia, V, Lattuada, N, Rapetti, D, et al. Ghrelin inhibits inflammatory pain in rats: Involvement of the opioid system. Neuropharmacology51, 497–505 (2006).
- ↵
Vergnano, AM, Ferrini, F, Salio, C, Lassi, L, Baratta, M and Merighi, A. The gastrointestinal hormone ghrelin modulates inhibitory neu-rotransmission
in deep laminae of mouse spinal cord dorsal horn. Endocrinology149, 2306–2312 (2008).
- ↵
Authier, F and Desbuquois, B. Glucagon receptors. Cell Mol Life Sci 65, 1880–1899 (2008).
-
Talhouk, RS, Saadé, NE, Mouneimne, G, Masaad, CA and Safieh-Garabedian, B. Growth hormone releasing hormone reverses endotoxin-induced
localized inflammatory hyperalgesia without reducing the upregulated cytokines, nerve growth factor and gelatinase activity.
Prog Neuropsychopharmacol Biol Psychiatry28, 625–631 (2004).
- ↵
Leal-Cerro, A, Povedano, J, Astorga, R, et al. The growth hormone (GH)-releasing hormone-GH-insulin-like growth factor-1 axis in patients
with fibromyalgia syndrome. J Clin Endocrinol Metab84, 3378–3381 (1999).
- ↵
Blackburn-Munro, G. Hypothalamo-pituitary-adrenal axis dysfunction as a contributory factor to chronic pain and depression. Curr Pain Headache Rep8, 116–124 (2004).
- ↵
Elwan, O, Abdella, M, el Bayad, AB and Hamdy, S. Hormonal changes in headache patients. J Neurol Sci106, 75–81 (1991).
- ↵
Hurley, RW and Adams, MC. Sex, gender, and pain: An overview of a complex field. Anesth Analg 107, 309–317 (2008).
- ↵
Raffa, RB. Antihistamines as analgesics. J Clin Pharm Ther26, 81–85 (2001).
- ↵
Kotani, M, Detheux, M, Vandenbogaerde, A, et al. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of
the orphan G protein–coupled receptor GPR54. J Biol Chem276, 34631–34636 (2001).
- ↵
Funk, CD. Prostaglandins and leukotrienes: Advances in eicosanoid biology. Science294, 1871–1875 (2001).
-
Shimizu, T. Lipid mediators in health and disease: enzymes and receptors as therapeutic targets for the regulation of immunity and inflammation.
Annu Rev Pharmacol Toxicol49, 123–150 (2009).
- ↵
Back, M. Functional characteristics of cysteinyl-leukotriene receptor subtypes. Life Sci71, 611–622 (2002).
- ↵
Park, KA and Vasko, MR. Lipid mediators of sensitivity in sensory neurons. Trends Pharmacol Sci 26, 571–577 (2005).
- ↵
Anliker, B and Chun, J. Cell surface receptors in lysophospholipid signaling. Semin Cell Dev Biol 15, 457–46 (2004).
- ↵
Ambriz-Tututi, M, Rocha-Gonzalez, HI, Cruz, SL, et al. Melatonin: A hormone that modulates pain. Life Sci84, 489–498 (2009).
- ↵
Starowicz, K and Przewlocka, B. The role of melanocortins and their receptors in inflammatory processes, nerve regeneration and nocicep-tion.
Life Sci 73, 823–847 (2003).
- ↵
Oertel, B and Lotsch, J. Genetic mutations that prevent pain: Implications for future pain medication. Pharmacogenomics 9, 179–194 (2008).
- ↵
Neugebauer, V. Metabotropic glutamate receptors—important modulators of nociception and pain behavior. Pain98, 1–8 (2002).
- ↵
Saito, Y and Maruyama, K. Identification of melanin-concentrating hormone receptor and its impact on drug discovery. J Exp Zoolog A Comp Exp Biol 305, 761–768 (2006).
- ↵
Sudo, H, Yoshida, S, Ozaki, K, et al. Oral administration of MA-2029, a novel selective and competitive motilin receptor antagonist,
inhibits motilin-induced intestinal contractions and visceral pain in rabbits. Eur J Pharmacol581, 296–305 (2008).
- ↵
Scarpignato, C and Pelosini, I. Management of irritable bowel syndrome: Novel approaches to the pharmacology of gut motility. Can J Gastroenterol 13, 50A–65A (1999).
- ↵
Dobner, PR. Neurotensin and pain modulation. Peptides27, 2405–2414 (2006).
- ↵
Torres, R, Croll, SD, Vercollone, J, et al. Mice genetically deficient in neuromedin U receptor 2, but not neuromedin U receptor 1,
have impaired nociceptive responses. Pain130, 267–278 (2007).
- ↵
Cao, CQ, Yu, XH, Dray, A, Filosa, A and Perkins, MN. A pro-nocice-ptive role of neuromedin U in adult mice. Pain104, 609–616 (2003).
- ↵
Xu, YL, Gall, CM, Jackson, VR, Civelli, O and Reinscheid, RK. Distribution of neuropeptide S receptor mRNA and neurochemical characteristics
of neuropeptide S-expressing neurons in the rat brain. J Comp Neurol500, 84–102 (2007).
- ↵
Reinscheid, RK, Xu, YL and Civelli, O. Neuropeptide S: A new player in the modulation of arousal and anxiety. Mol Interv5, 42–46 (2005).
- ↵
Hokfelt, T, Brumovsky, P, Shi, T, Pedrazzini, T and Villar, M. NPY and pain as seen from the histochemical side. Peptides28, 365–372 (2007).
- ↵
Smith, PA, Moran, TD, Abdulla, F, Tumber, KK and Taylor, BK. Spinal mechanisms of NPY analgesia. Peptides28, 464–474 (2007).
- ↵
Hondo, M, Ishii, M and Sakurai, T. The NPB/NPW neuropeptide system and its role in regulating energy homeostasis, pain, and emotion.
Results Probl Cell Differ46, 239–256 (2008).
-
Yamamoto, T, Saito, O, Koyo, S and Tanabe, S. Anti-hyperalgesic effects of intrathecally administered neuropeptide W-23, and neuropep-tide
B, in tests of inflammatory pain in rats. Brain Res1045, 97–106 (2005).
- ↵
Kelly, MA, Beuckmann, CT, Williams, SC, et al. Neuropeptide B-deficient mice demonstrate hyperalgesia in response to inflammatory pain.
Proc Natl Acad Sci USA102, 9942–9947 (2005).
- ↵
Yang, HY, Tao, T and Iadarola, MJ. Modulatory role of neuropeptide FF system in nociception and opiate analgesia. Neuropeptides42, 1–18 (2008).
- ↵
Yang, HY and Iadarola, MJ. Modulatory roles of the NPFF system in pain mechanisms at the spinal level. Peptides 27, 943–952 (2006).
- ↵
Soudijn, W, van Wijngaarden, I and Ijzerman, AP. Nicotinic acid receptor subtypes and their ligands. Med Res Rev27, 417–433 (2007).
- ↵
Trescot, AM, Helm, S, Hansen, H, et al. Opioids in the management of chronic non-cancer pain: An update of American Society of the Interventional
Pain Physicians’ (ASIPP) Guidelines. Pain Physician 11, S5–S62 (2008).
- ↵
Donnelly-Roberts, D, McGaraughty, S, Shieh, CC, Honore, P and Jarvis, MF. Painful purinergic receptors. J Pharmacol Exp Ther324, 409–415 (2008).
- ↵
Tozaki-Saitoh, H, Tsuda, M, Miyata, H, Ueda, K, Kohsaka, S and Inoue, K. P2Y12 receptors in spinal microglia are required for neuropathic
pain after peripheral nerve injury. J Neurosci28, 4949–4956 (2008). This study indicates the importance of nucleotide-activated GPCRs in the activation of glial responses to nerve injury
that play a critical role in the development of chronic neuropathic pain. The results underscore the importance of glial mechanisms
in persistent pain and the potential value of glial GPCRs as therapeutic targets..
- ↵
Nagae, M, Hiraga, T, Wakabayashi, H, Wang, L, Iwata, K and Yoneda, T. Osteoclasts play a part in pain due to the inflammation adjacent
to bone. Bone39, 1107–1115 (2006).
- ↵
Morita, K, Morioka, N, Abdin, J, Kitayama, S, Nakata, Y and Dohi, T. Development of tactile allodynia and thermal hyperalgesia by intrathe-cally
administered platelet-activating factor in mice. Pain111, 351–359 (2004).
- ↵
Teather, LA, Magnusson, JE and Wurtman, RJ. Platelet-activating factor antagonists decrease the inflammatory nociceptive response in
rats. Psychopharmacology (Berl)163, 430–433 (2002).
- ↵
Negri, L, Lattanzi, L, Giannini, E and Melchiorri, P. Bv8/Prokineticin proteins and their receptors. Life Sci81, 1103–1116 (2007).
-
Vellani, V, Colucchi, M, Lattanzi, R, et al. Sensitization of transient receptor potential vanilloid 1 by the prokineticin receptor
agonist Bv8. J Neurosci26, 5109–5116 (2006).
- ↵
Negri, L, Lattanzi, R, Giannini, E, et al. Impaired nociception and inflammatory pain sensation in mice lacking the prokineticin receptor
PKR1: Focus on interaction between PKR1 and the capsaicin receptor TRPV1 in pain behavior. J Neurosci26, 6716–6727 (2006).
- ↵
Hollenberg, MD and Compton, SJ. International Union of Pharmacology. XXVIII. Proteinase-activated receptors. Pharmacol Rev 54, 203–217 (2002).
-
Russo, A, Soh, UJK and Trejo, J. Proteases display biased agonism at protease-activated receptors: Location matters!. Mol Interv9, 168–170 (2009).
- ↵
Russell, FA and McDougall, JJ. Proteinase activated receptor (PAR) involvement in mediating arthritis pain and inflammation. Inflamm Res 58, 119–126 (2009).
- ↵
Kalliomaki, ML, Petrovaara, A, Brandt, A, et al. Prolactin-releasing peptide affects pain, allodynia and autonomic reflexes through medullary
mechanisms. Neuropharmacology46, 412–424 (2004).
- ↵
Kristiansson, P, Svardsudd, K and von Schoultz, B. Serum relaxin, symphyseal pain, and back pain during pregnancy. Am J Obstet Gynecol175, 1342–1347 (1996).
- ↵
Carlton, SM, Zhou, S, Du, J, Hargett, G, Ji, G and Coggeshall, R. Somatostatin modulates the transient receptor potential vanilloid 1
(TRPV1) ion channel. Pain110, 616–627 (2004). This study indicates that Gi protein–coupled somatostatin receptors expressed in peripheral nociceptors inhibit nociceptive signalling and reduce pain
behavior. Furthermore, it provides evidence that nociceptive signaling (in this case the action of TRPV1) is under tonic inhibitory
regulation by Gi protein–coupled receptors..
- ↵
Pinter, E, Helyes, Z and Szolcsanyi, J. Inhibitory effect of somatostatin on inflammation and nociception. Pharmacol Ther112, 440–456 (2006).
- ↵
Mantyh, PW. Neurobiology of substance P and the NK1 receptor. J. Clin. Psychiatry63Suppl_11, 6–11 (2002).
- ↵
Hill, R. NK1 (substance P) receptor antagonists--why are they not analgesic in humans. Trends Pharmacol Sci21, 244–246 (2000).
- ↵
Tanabe, M, Tokuda, Y, Takas, UK, Ono, K, Honda, M and Ono, H. The synthetic TRH analogue taltirelin exerts modality-specific antinociceptive
effects via distinct descending monoaminergic systems. Br J Pharmacol150, 403–414 (2007).
- ↵
Lindemann, L and Hoener, MC. A renaissance in trace amines inspired by a novel GPCR family. Trends Pharmacol Sci 26, 274–281 (2005). This review discusses the recently identified family of trace amine binding GPCRs. Trace amines are present in the CNS
at low levels and are typically metabolic products of the biogenic amine neu-rotransmitters. Although trace amines had been
previously linked to neurological disorders, their mechanism of action was unknown. The discovery of this novel family of
GPCRs suggests a role for trace amines as neurotransmitters or neuromodulators; these receptors are prime candidates for new
drug discovery..
- ↵
D’Andrea, G, Terrazzino, S, Leon, A, et al. Elevated levels of circulating trace amines in primary headaches. Neurology62, 1701–1705 (2004).
- ↵
Do-Rego, JC, Chatenet, D, Orta, MH, et al. Behavioral effects of urotensin-II centrally administered in mice. Psychopharmacology (Berl)183, 103–117 (2005).
- ↵
Honda, K and Takano, Y. New topics in vasopressin receptors and approach to novel drugs: Involvement of vasopressin V1a and V1b receptors
in nociceptive responses and morphine-induced effects. J Pharmacol Sci 109, 38–43 (2009).
-
Koshimizu, TA and Tsujimoto, G. New topics in vasopressin receptors and approach to novel drugs: Vasopressin and pain perception. J Pharmacol Sci 109, 33–37 (2009).
- ↵
Zingg, HH and Laporte, SA. The oxytocin receptor. Trends Endocrinol Metab 14, 222–227 (2003).
- ↵
Dickinson, T and Fleetwood-Walker, SM. VIP and PACAP: very important in pain. Trends Pharmacol Sci 20, 324–329 (1999).
-
Jongsma, H, Pettersson, LM, Zhang, YZ, et al. Markedly reduced chronic nociceptive response in mice lacking the PAC1 receptor. Neuroreport12, 2215–2219 (2001).
- ↵
Mabuchi, T, Shintani, N, Matsumara, S, et al. Pituitary adenylate cycla-se-activating polypeptide is required for the development of
spinal sensitization and induction of neuropathic pain. J Neurosci24, 7283–7291 (2004).
Laura S. Stone, PhD, received her doctoral degree at the University of Minnesota and postdoctoral training at the Oregon Health and Sciences
University (OHSU). Following a brief interlude in biotechnology, she returned to academia and is currently an Assistant Professor
in the Faculty of Dentistry at McGill University. Her research program utilizes both human and pre-clinical models to study
synergistic interactions among GPCRs in pain and analgesia. Her work also concerns the etiology and treatment of chronic low
back pain. Send correspondence to LSS. Email laura.s.stone{at}mcgill.ca; fax 514-398-7203.
Derek C. Molliver, PhD, received his doctoral degree at Washington University in St. Louis and postdoctoral training at the Oregon Health and Science
University (OHSU). He is currently an Assistant Professor in the Departments of Medicine and Neurobiology at the University
of Pittsburgh and a member of the Pittsburgh Center for Pain Research. His interests are focused on mechanisms underlying
the regulation of sensory neuron development and functional plasticity by G protein–coupled receptors and neurotrophic factors.