
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
Wishing Away Inflammation? New Links between Serotonin and TNF Signaling
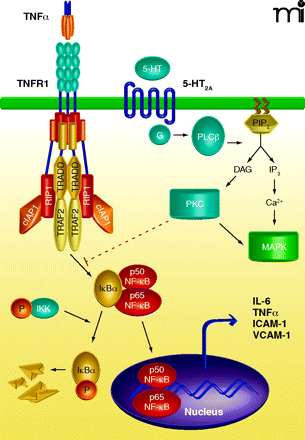
Potential mechanism of crosstalk between the tumor necrosis factor receptor 1 (TNFR1) and serotonin subtype 2A (5-HT2A) receptor signaling. The primary TNFR1 signaling complex consists of the adapter protein TRADD, the ring finger–containing protein TRAF2, the inhibitor of apoptosis protein cIAP1, and the protein kinase RIP1. Following TNF binding, the receptor complex activates the IκB kinase complex to trigger IκB degradation and the release of NF-κB subunits into the nucleus where they function as transcription factors. The TNF-bound receptor complex also induces the expression of several pro-inflammatory genes such as IL-6, TNFα, ICAM-1, and VCAM-1. The receptor for serotonin, 5-HT2A is a G protein–coupled receptor able to stimulate phospholipase C (PLC), a membrane-bound enzyme that catalyzes the degradation of the inositol lipid phosphatidylinositol 4,5-bisphosphate (PIP2), producing inositol 1,4,5-triphosphate (IP3) and diacylglycerol (DAG). IP3 mobilizes Ca2+ that induces multiple responses in the cell, including activation of mitogen-activated protein kinase (MAPK), while DAG activates another kinase family, protein kinase C (PKC). Results from Yu and colleagues (3) suggest that 5-HT2A receptor-mediated anti-inflammatory effects are mediated through activation of PKC, which acts most probably at a level proximal to NF-κB nuclear translocation. TRADD, TNFR1-associated death domain protein; TRAF2, TNF receptor-associated factor 2; cIAP1, cellular inhibitor of apotosis 1; RIP, receptor-interacting serine–threonine protein kinase; IL-6, interleukin-6; ICAM-1, intercellular adhesion molecule-1; VCAM- 1, vascular cell adhesion molecule-1.


