|
 
 |
| ORIGINAL ARTICLE |
|
| Year : 2016 | Volume
: 4
| Issue : 1 | Page : 41-46 |
|
Rauvolfia vomitoria and Gongronema latifolium stimulate cortical cell proliferations
Moses B Ekong1, Ubong U Ekpene2, Agnes A Nwakanma3, Aniekan I Peter1, Bassey T Etuknwa1
1 Department of Anatomy, Faculty of Basic Medical Sciences, University of Uyo, Uyo, Nigeria
2 Department of Surgery, University of Uyo Teaching Hospital, Uyo, Nigeria
3 Department of Anatomy, Faculty of Basic Medical Sciences, Chukwuemeka Odumegwu Ojukwu University, Uli, Nigeria
| Date of Web Publication | 13-Sep-2016 |
Correspondence Address:
Moses B Ekong
Department of Anatomy, Faculty of Basic Medical Sciences, University of Uyo, Uyo
Nigeria
 Source of Support: None, Conflict of Interest: None  | Check |
DOI: 10.4103/2315-7992.190464

Introduction: Rauwolfia vomitoria (RV) and Gongronema latifolium (Gl) are herbs with closely related and diverse medicinal properties. The combination of both plants is reported to have the potentials for brain functions and structure protection. Aim: This study therefore investigated the interaction of these herbs on the histomorphology of the cerebral cortex of mice. Materials and Methods: 24 male Wistar mice of body weight 15-26 g were divided into 4 groups. The mice were administered respectively, 0.5 mL of Tween 20, 150 mg/kg of R. vomitoria, 200 mg/kg of G. latifolium, and a combination of 150 mg/kg of R. vomitoria and 200 mg/kg of G. latifolium (RV+GL), orally, and daily for seven days. On day 8, the animals were sacrificed and their brains preserved, and the cerebral cortices were excised for routine histology. Cellular densities were quantified using ImageJ™. Results: All the groups gained body weight, which was however lower in the test groups compared with the control group. No difference was observed in whole brain weight in all the experimental groups, while histomorphological studies of the cerebral cortex showed higher cellular density and smaller cellular sizes in the RV, GL and RV+GL groups. The RV+GL group also showed slightly larger cells in the cortical plate compared with the control group. The mean cellular population of sections of the cerebral cortex were also higher in test groups RV and GL, but not the RV+GL group. Conclusion: This study showed that R. vomitoria root bark and G. latifolium leaf extracts either singly or in combination may stimulate cellular proliferation at the given dose, which may serve a protective or deleterious role. Keywords: Brain, cerebral cortex, Gongronema latifolium, mice, Rauvolfia vomitoria
How to cite this article:
Ekong MB, Ekpene UU, Nwakanma AA, Peter AI, Etuknwa BT. Rauvolfia vomitoria and Gongronema latifolium stimulate cortical cell proliferations. Ann Bioanthropol 2016;4:41-6 |
How to cite this URL:
Ekong MB, Ekpene UU, Nwakanma AA, Peter AI, Etuknwa BT. Rauvolfia vomitoria and Gongronema latifolium stimulate cortical cell proliferations. Ann Bioanthropol [serial online] 2016 [cited 2017 Apr 6];4:41-6. Available from: http://www.bioanthrojournal.org/text.asp?2016/4/1/41/190464 |
| Introduction | |  |
The rising cost of modern and more efficacious orthodox drugs, its availability and safety have led to the patronage of alternative medication.[1] Most of these alternatives are herbs, and reports show that these herbs have wide ranges of useful medicinal properties.[2],[3],[4] Herbs such as Rauvolfia vomitoria (RV) and Gongronema latifolium (GL) have closely related, as well as diverse properties,[5],[6],[7],[8],[9] and these have led to their medicinal uses.
RV belongs to the family Apocynaceae, and it is commonly called serpent wood or swizzle stick. In Nigerian languages, it is known as eto mmong eba ebot in Ibibio, akanta in Igbo, asofeyeje in Yoruba, and wada in Hausa. RV contains alkaloid which includes ajmaline, ajmalicine, reserpine, serpentine, serpentinine, and yohimbine among others.[3],[10] This herb has been reported to improve immunity and restore hematologic parameters.[11] Analgesic, anticonvulsant, and antipsychotic effects have been reported,[5],[12],[13] while it was previously reported that RV restores brain mental activities.[14]
Side effects of RV in animal models have been reported: In the fetus, hepatotoxicity, cardiotoxicity, hypertrophy, and hyperplasia of osteoblasts and osteoclasts in the femur have been reported.[15],[16],[17] Depression and Parkinsonism More Details,[10] degenerative changes in cerebellar and cerebral cortical cytoarchitectures, and neurobehaviors have also been reported in adults.[18],[19],[20],[21],[22]
RV causes sedation,[18],[19],[22] which if not controlled may result in death due to appetite loss. The effectiveness of RV in combination with other herbs such as GL has been reported with minimal side effects;[19],[20],[21],[22],[23] hence, this study is to investigate if the combination herbs could be beneficial to the cerebral cortical morphology.
GL belongs to the family Asclepiadaceae, and it is commonly called amaranth globe and bush buck. In Nigerian languages, it is known as utasi in Ibibio, utazi in Igbo, and arokeke in Yoruba.[8] The plant is used for both medicinal and nutritional purposes.[24],[25] GL contains alkaloids, saponins, tannins, flavonoids, and glycosides.[25],[26] Properties such as hypoglycemic, antibacterial, antioxidant, anti-inflammatory, antiulcer, analgesic, antidiabetic, and antipyretic activities have been reported.[4],[7],[9],[25],[27],[28] GL is also reported to be a bittering agent, as well as having cardioprotective properties.[6],[29]
Both plants show potentials for brain function improvement and protection [13],[22],[23] although a recent report showed no effect on spatial learning and memory, as well as some biomolecules and enzyme activities.[23] Therefore, this investigation was to investigate the combined properties of these herbs on the histology of the cerebral cortex of mice.
| Materials and Methods | |  |
Thirty male Wistar mice of age 3 months and body weight 15–26 g obtained from the animal facility of the Faculty of Basic Medical Sciences of the University of Uyo, Nigeria, were handled in accordance with the international guidelines for the use and care of laboratory animals. The animals were provided with normal mice chow (Vital Feed, Jos, Nigeria) and water ad libitum throughout the duration of the experiment.
Preparation of herbs
Fresh leaves and roots of GL and RV were, respectively, obtained from local farms in Ika and Esit Eket in Akwa Ibom State of Nigeria. Samples of the plants were authenticated by a botanist in the Department of Botany of the University. The root barks were separated from the cambium, and together with the leaves were cleaned of impurities. The parts of these plants were air-dried and crushed to fine powder and were macerated in 75–80% ethanol, using Soxhlet extractor. The extracts were concentrated using a rotary evaporator, and the concentrates were dried in a Plus 11 Gallenkamp oven at 45–50°C. The dry extracts obtained were stored in a refrigerator at 4°C until used. Tween 20 was used as the vehicle to dissolve the extracts. Two gram of each extract was dissolved in 30 mL of Tween 20, and the actual dosages were calculated.
Experimental protocol
The mice were weighed and divided into four groups of six mice each. Animals in Group 1 (control) were administered 0.5 mL of Tween 20. Group 2 animals were administered 150 mg/kg of RV. Group 3 animals were administered 200 mg/kg of GL, whereas Group 4 animals were administered a combination of 150 mg/kg of RV and 200 mg/kg of GL. All the treatments were by oral gavages, carried out at 8 am every day for 7 days [Table 1].
Twenty-four hours after the last administrations, the animals were anesthetized using chloroform fumes and were humanely sacrificed. The brains were removed, weighed, and preserved in 10% buffered formalin for 7 days. Each cerebral cortex was excised, routinely processed and stained following the hematoxylin and eosin staining method. The nuclei of the cells were quantified using ImageJ™ software (version 1.37c, NIH, USA) with the aid of a computer operated digital camera linked to the microscope to determine the cellular population, and photomicrographs of the sections were obtained.
Statistical analysis
One-way analysis of variance was used to compare the means for treatment and their interactions; thereafter, the post hoc test using Student–Newman–Keuls multiple comparisons test was carried out to find the level of significance at P < 0.05. All the results were expressed as mean ± standard deviation.
| Results | |  |
Weight changes study
There was gradual increase in body weights in all the experimental groups, but from day 6 of the administration of the extracts, there was loss of body weights in the test groups which received 150 mg/kg of RV and 200 mg/kg of GL either alone or in combination (RV + GL), compared with the control group, which was increased during the same period [Figure 1]. The percentage increase in body weights during the experimental period for the RV, GL, and RV + GL groups were 4.50%, 3.88%, and 3.28%, respectively, compared with the control group (28.57%) [Table 2].
There was no difference in the whole brain weight of all the test groups compared with the control group. The mean cellular population in sections of the cerebral cortex were significantly (P < 0.05) higher in the RV and GL groups, but not the RV + GL group compared with the control. However, the cellular population of the RV + GL was significantly (P < 0.05) lower than the RV and GL groups [Table 3].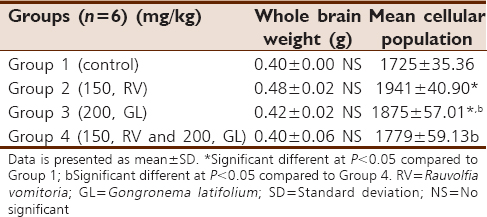 | Table 3: Whole brain weight and cellular population of the cerebral cortex of the mice
Click here to view |
Histomorphological study
The histological section of the cerebral cortex of the mice of the control group showed six cortical layers; from the superficial inwards were marginal zone, cortical plate, subcortical plate, intermediate, subventricular, and the ventricular zones. The marginal zone consisted mostly of fibers with sparse cell density. The cortical plate showed much cell density. The subcortical plate had less cellular density. The intermediate, subventricular, and the ventricular zones were not distinguishable, with the layers showing numerous cells [Figure 2].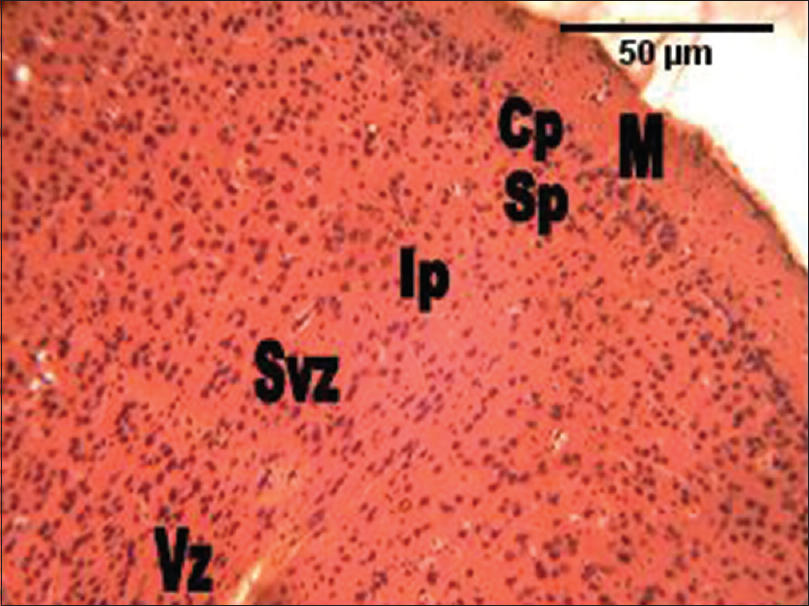 | Figure 2: The photomicrograph of the cerebral cortex of the control group showing the marginal zone containing mostly processes, with sparse cell density. The cortical plate shows much cell density. The subcortical plate has less cellular density. The intermediate, subventricular and the ventricular zones are not distinguishable, with the layers showing numerous cellular densities. M = Marginal zone; Cp = Cortical plate; Sp = Subcortical plate; Ip = Intermediate; Svz = Sub-ventricular; and the Vz = Ventricular zones (H and E, ×100)
Click here to view |
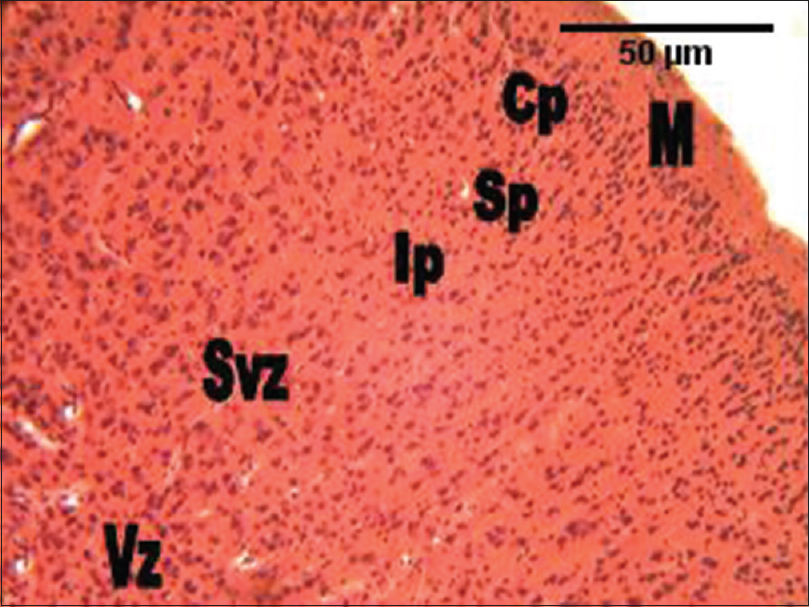
In the group administered RV alone, the histological section of the cerebral cortex showed more cellular density, and smaller cellular sizes compared with the control group. Every other area of the section appeared like the control [Figure 3]. In the group administered GL alone, the histological section of the cerebral cortex also showed much cellular density and smaller cellular sizes in all the cortical layers compared with the control group. Every other area of the section appeared like the control [Figure 4]. In the group administered the combination of RV and GL (RV + GL), the histological section of the cerebral cortex showed much cellular density as well, but smaller cellular sizes in the intermediate zones through to the ventricular zones. However, the cortical cells were slightly larger compared with the control group [Figure 5].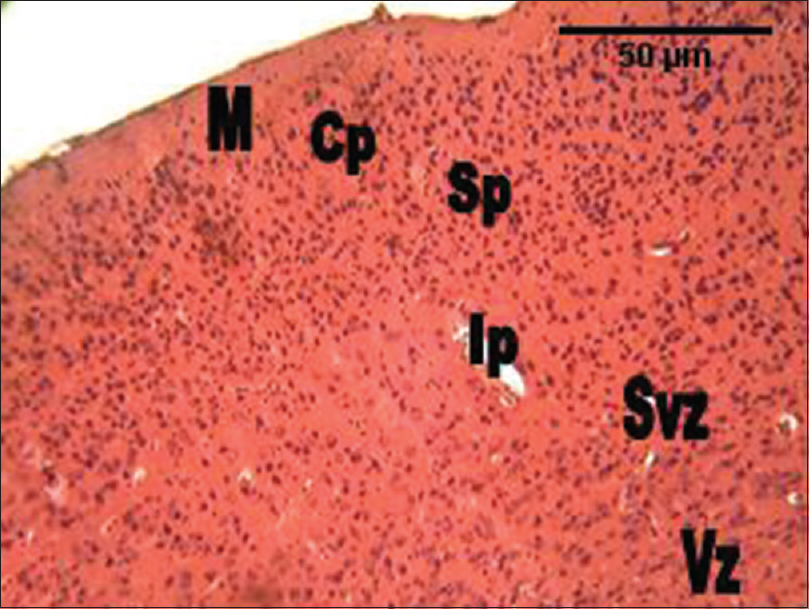 | Figure 3: The photomicrograph of the cerebral cortex of the group administered Rauvolfia vomitoria (RV) alone showing more cellular density, and smaller cellular sizes compared with the control group. M = Marginal zone; Cp = Cortical plate; Sp = Subcortical plate; Ip = Intermediate; Svz = Sub-ventricular; and the Vz = Ventricular zones (H and E, ×100)
Click here to view |
 | Figure 4: The photomicrograph of the cerebral cortex of the group administered Gongronema latifolium alone showing much cellular density and smaller cellular sizes in all the cortical layers compared with the control group. M = Marginal zone; Cp = Cortical plate; Sp = Subcortical plate; Ip = Intermediate; Svz = Subventricular; and the Vz = Ventricular zones (H and E, ×100)
Click here to view |
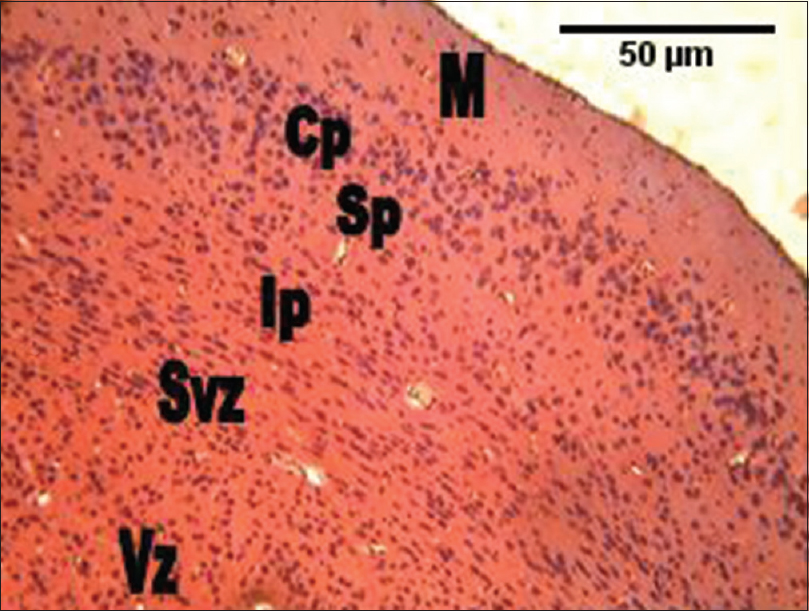 | Figure 5: The photomicrograph of the cerebral cortex of the group administered the combination of Rauvolfia vomitoria and Gongronema latifolium (Rauvolfia vomitoria+ Gongronema latifolium) showing much cellular density, but smaller cellular sizes in the intermediate zones through to the ventricular zones. However, the cortical cells were slightly larger compared with the control group. M = Marginal zone; Cp = Cortical plate; Sp = Subcortical plate; Ip = Intermediate; Svz = Sub-ventricular; and the Vz = Ventricular zones (H and E, ×100)
Click here to view |
| Discussion | |  |
Most alternative medicine practitioners use herbs without taking into consideration the potent adverse effects that may result from such. RV has been reported to have adverse effects added to its useful properties.[17],[18],[19] As such, the combination with other equally useful herbs may modulate the adverse effects while still maintaining its useful abilities;[19] thus, the use of RV in combination with GL in this study.
All the groups gained body weight in the course of the experiment. However, there was a loss in body weights in the test group animals from day 6 of the experiment. The gain in body weight was lower in the test groups compared with the control group. There are reports that individual administration of RV and GL result in body weight loss in rats.[18],[19],[22],[30],[31] However, no difference in body weights was reported when mice were administered same.[20],[22] The present study may be due to the administered dose and the specie of the animal and is at variance with previous reports.
GL was previously reported as a traditional tool in the control of body weight gain by lactating women.[32] While groups administered either RV or GL alone or their combination did show body weight gain, it appeared controlled compared with the control group. These results further buttress that RV and GL may be useful in body weight gain control.
Morphometric studies showed no difference in whole brain weight in all the experimental groups, an indication that the size of the brains of the animals was not affected by the administered substances which are in line with previous studies.[20],[21] Histomorphological and morphometrical study of the cerebral cortex showed higher cellular density, and smaller cellular sizes in the RV and GL groups though the RV + GL group cellular density was however not significant, but showed much cellular, but smaller cellular sizes in the intermediate zones through to the ventricular zones, and the cortical cells were slightly larger compared with the control group.
The histomorphology result of this study indicates that the extracts either singly or in combination have the potential to stimulate cellular proliferation in the cerebral cortex. The reported cellular proliferation may be due to either gliosis and/or neurogenesis. Gliosis usually result when the brain is traumatized by chemical agents and/or infections,[33],[34] as in this case, the administered substances. It is reported that reserpine, which is the most abundant and most active alkaloid in RV have both depressive and antidepressant effects on the nervous system.[35],[36] Antidepressants have been reported to increase neurogenesis in the adult rodent hippocampus.[37],[38],[39] It is possible that neurogenesis may be stimulated as well in the cerebral cortex in this study, as previous studies have also shown that neurogenesis occurs in the cerebellum of rabbits.[40],[41]
The cerebral cortex is the outer layer of mammalian cerebrum and plays a major role in memory, attention, perception, awareness, thought, language, and consciousness.[42] The morphological changes in the cerebral cortex as reported in this study may be due to plasticity usually associated with morphological changes in neuronal distribution.[43] While this morphological changes may be beneficial for normal function and for compensatory recovery,[44] it may also be deleterious,[45] resulting in the inhibition of the processing abilities of the cortex.
| Conclusion | |  |
This study showed that RV root bark and GL leaf extracts either singly or in combination may stimulate cellular proliferation at the given dose, which may serve either a protective or deleterious role.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| References | |  |
| 1. | Moody JO. Traditional Medicine. Paper Delivered at the Mandatory Continuing Professional Development (MCPD) Programme, Module V, Faculty of Pharmacy, University of Ibadan; 21-22 November, 2007. p. 1-6.  |
| 2. | Sofowora A. Medicinal Plant and Traditional Medicine in Africa. 2 nd ed. Ibadan: Spectrum Books Limited; 1993. p. 1-153.  |
| 3. | Sharma R. Agro-Techniques of Medicinal Plants. New Delhi: Daya Publishing House; 2004. p. 3-8.  |
| 4. | Okokon JE, Umoh UF, Ekpo BA, Etim EI. Antidiabetic study of combined extracts of Vernonia amygdalina, Ocimum gratissimum, and Gongronema latifolium on alloxan-induced diabetic rats. J Nat Pharm 2013;4:28-31.  |
| 5. | Amole OO, Onabanjo AO, Odofin AA. The analgesic effect of Rauvolfia vomitoria (Afzel). Biomed Res 2006;17:125-7.  |
| 6. | Adenuga W, Olaleye ON, Adepoju PA. Utilization of bitter vegetable leaves ( Gongronema latifolium, Vernonia amygdalina) and Garcinia kola extracts as substitutes for hops in sorghum beer production. Afr J Biotechnol 2010;9:8819-23.  |
| 7. | Akuodor GC, Idris-Usman MS, Mbah CC, Megwas UA, Akpan JL, Ugwu TC, et al. Studies on anti-ulcer, analgesic and antipyretic properties of the ethanolic leaf extract of Gongronema latifolium in rodents. Afr J Biotechnol 2010;9:2316-21.  |
| 8. | |
| 9. | Ugochukwu NH, Babady NE, Cobourne M, Gasset SR. The effect of Gongronema latifolium extracts on serum lipid profile and oxidative stress in hepatocytes of diabetic rats. J Biosci 2003;28:1-5.  |
| 10. | Okpako DT. Principles of Pharmacology: A Tropical Approach. New York: Cambridge University Press; 1991. p. 35-60.  |
| 11. | Isaiah AM, Olawale O, Effiong EE, Idongesit NJ, Fidelis UA, Friday UU. Vitamin E supplementation with Rauvolfia vomitoria root bark extract improves hematological indices. N Am J Med Sci 2012;4:86-9.  |
| 12. | Amole OO, Yemitan OK, Oshikoya KA. Anticonvulsant activity of Rauvolfia vomitoria (Afzel). Afr J Pharm Pharmacol 2009;3:319-22.  |
| 13. | Bisong S, Brown R, Osim E. Comparative effects of Rauvolfia vomitoria and chlorpromazine on social behaviour and pain. N Am J Med Sci 2011;3:48-54.  |
| 14. | Lambo JO. Management of hypertension in traditional medicine. In: Sofowora A, editor. Antihypertension Agents from National Sources. Ile-Ife; University of Ife Press; 1975. p. 23-45.  |
| 15. | Eluwa MA, Ekanem TB, Udoh PB, Akpantah AO, Ekong MB, Asuquo OR, et al. Teratogenic effect of crude ethanolic root bark and leaf extracts of Rauvolfia vomitoria ( Apocynaceae) on the liver of albino Wistar rat fetuses. Asian J Med Sci 2013;4:30-4.  |
| 16. | Eluwa MA, Ekanem TB, Udoh PB, Ekong MB, Akpantah AO, Asuquo OA, et al. Teratogenic effects of crude ethanolic root bark and leaf extracts of Rauvolfia vomitoria ( Apocynaceae) on the femur of albino Wistar rat fetuses. J Histol 2013b; 2013:1-5.  |
| 17. | Eluwa MA, Udoaffah MT, Vulley MB, Ekanem TB, Akpantah AO, Asuquo OA, et al. Comparative study of teratogenic potentials of crude ethanolic root bark and leaf extract of Rauvolfia vomitoria ( Apocynaceae) on the fetal heart. N Am J Med Sci 2010;2:592-5.  |
| 18. | Eluwa MA, Idumesaro NB, Ekong MB, Akpantah AO, Ekanem TB. Effect of aqueous extract of Rauvolfia vomitoria root bark on the cytoarchitecture of the cerebellum and neurobehaviour of adult male Wistar rats. Internet J Alternat Med 2009;6.  |
| 19. | Ekong MB, Peter AI, Davies K, Bassey EI, Aquaisua AN, Akpanabiatu MI, et al. Gongronema latifolium ameliorates Rauvolfia vomitoria induced behavior, biochemicals, and histomorphology of the cerebral cortex. J Neurochem 2013;125 Suppl 1:369.  |
| 20. | Ekong MB, Peter MD, Peter AI, Eluwa MA, Umoh IU, Igiri AO, et al. Cerebellar neurohistology and behavioural effects of Gongronema latifolium and Rauvolfia vomitoria in mice. Metab Brain Dis 2014;29:521-7.  |
| 21. | Ekong MB, Ekpene UU, Thompson FE, Peter AI, Udoh NB, Ekandem GJ. Effects of co-treatment of Rauvolfia vomitoria and Gongronema latifolium on neurobehaviour and the neurohistology of the cerebral cortex in mice. Internet J Med Update 2015;10:3-10.  |
| 22. | Ekong MB, Peter AI, Ekpene UU, Bassey EI, Eluwa MA, Akpanabiatu MI, et al. G. latifolium modulates R. vomitoria induced behaviour and histomorphology of the cerebral cortex. Int J Morphol 2015;33:77-84.  |
| 23. | Ekong MB, Peter AI, Ekpene UU. Co-administration of Rauvolfia vomitoria with Gongronema latifolium or Vernonia amygdalina on spatial learning, memory, and some bio-molecules. Asian J Med Sci 2016;7:82-7.  |
| 24. | Agbo CU, Baiyeri KP, Obi IU. Indigenous knowledge and utilization of Gongronema latifolia Benth: A case study of women in University of Nigeria, Nsukka. Biores J 2005;3:66-9.  |
| 25. | Eleyinmi AF. Chemical composition and antibacterial activity of Gongronema latifolium. J Zhejiang Univ Sci B 2007;8:352-8.  |
| 26. | Nwinyi OC, Chinedu NS, Ajani OO. Evaluation of antibacterial activity of Psidium guajava and Gongronema latifolium. J Med Plants Res 2008;2:189-92.  |
| 27. | Morebise O, Fafunso MA, Makinde JM, Olajide OA, Awe EO. Antiinflammatory property of the leaves of Gongronema latifolium. Phytother Res 2002;16 Suppl 1:S75-7.  |
| 28. | Ogundipe OO, Moody JO, Akinyemi TO, Raman A. Hypoglycemic potentials of methanolic extracts of selected plant foods in alloxanized mice. Plant Foods Hum Nutr 2003;58:1-7.  |
| 29. | Glew RS, Vander Jagt DJ, Huang YS, Chuang LT, Bosse R, Glew RH. Nutritional analysis of the edible pit of Sclerocarya birrea in the Republic of Niger (daniya, Hausa). J Food Compost Anal 2004;17:99-111.  |
| 30. | Effiong GS, Udoh IE, Mbagwu HO, Ekpe IP, Asuquo EN, Atangwho IJ, et al. Acute and chronic toxicity studies of the ethanol leaf extract of Gongronema latifolium. Int Res J Biochem Bioinform 2012;2:155-61.  |
| 31. | Ezeonwu VU. Effects of Ocimum gratissimum and Gongronema latifolium on fertility parameters: A case for bi-herbal formulations. Stand Res J Med Plants 2013;1:1-5.  |
| 32. | Schneider CR, Sheidt K, Brietmaier E. Four new pregnan glycosides from Gongronema latifolium ( Asclepiadaceae). J Parkt Chem Chem Ztg 2003;353:532-6.  |
| 33. | Roumier A, Pascual O, Béchade C, Wakselman S, Poncer JC, Réal E, et al. Prenatal activation of microglia induces delayed impairment of glutamatergic synaptic function. PLoS One 2008;3:e2595.  |
| 34. | Tambuyzer BR, Ponsaerts P, Nouwen EJ. Microglia: Gatekeepers of central nervous system immunology. J Leukoc Biol 2009;85:352-70.  |
| 35. | Iversen LL, Glowinski J, Axelrod J. The uptake and storage of H3-norepinephrine in the reserpine-pretreated rat heart. J Pharmacol Exp Ther 1965;150:173-83.  |
| 36. | López-Muñoz F, Bhatara VS, Alamo C, Cuenca E. Historical approach to reserpine discovery and its introduction in psychiatry. Actas Esp Psiquiatr 2004;32:387-95.  |
| 37. | Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci 2000;20:9104-10.  |
| 38. | Chen G, Rajkowska G, Du F, Seraji-Bozorgzad N, Manji HK. Enhancement of hippocampal neurogenesis by lithium. J Neurochem 2000;75:1729-34.  |
| 39. | Sairanen M, Lucas G, Ernfors P, Castrén M, Castrén E. Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J Neurosci 2005;25:1089-94.  |
| 40. | Ponti G, Peretto P, Bonfanti L. A subpial, transitory germinal zone forms chains of neuronal precursors in the rabbit cerebellum. Dev Biol 2006;294:168-80.  |
| 41. | Ponti G, Peretto P, Bonfanti L. Genesis of neuronal and glial progenitors in the cerebellar cortex of peripuberal and adult rabbits. PLoS One 2008;3:e2366.  |
| 42. | Kandel ER, Hudspeth AJ. The brain and behavior. In: Kandel ER, Schwartz JH, Jessell TM, Siegelbaum SA, Hudspeth AJ, editors. Principles of Neural Science. 5 th ed. New York: McGraw-Hill Education/Medical; 2012. p. 5-19.  |
| 43. | Donoghue JP, Hess G, Sanes JN. Substrates and mechanisms for learning in motor cortex. In: Bloedel J, Ebner T, Wise SP, editors. Acquisition of Motor Behavior in Vertebrates. Cambridge, MA: MIT Press; 1996. p. 363-86.  |
| 44. | Buonomano DV, Merzenich MM. Cortical plasticity: From synapses to maps. Annu Rev Neurosci 1998;21:149-86.  |
| 45. | Cohen LG, Ziemann U, Chen R, Classen J, Hallett M, Gerloff C, et al. Studies of neuroplasticity with transcranial magnetic stimulation. J Clin Neurophysiol 1998;15:305-24.  |
[Figure 1], [Figure 2], [Figure 3], [Figure 4], [Figure 5]
[Table 1], [Table 2], [Table 3]
|