|
 
 |
| ORIGINAL ARTICLE |
|
| Year : 2016 | Volume
: 4
| Issue : 2 | Page : 84-89 |
|
Murine's lateral frontal cortical histomorphology and its behavior after caffeine administration
Moses Bassey Ekong1, Eno-Obong Henrietta Akpan1, Agnes Akudo Nwakanma2
1 Department of Anatomy, Faculty of Basic Medical Sciences, University of Uyo, Uyo, Nigeria
2 Department of Anatomy, Faculty of Basic Medical Sciences, Chukwuemeka Odumegwu Ojukwu University, Uli, Anambra, Nigeria
| Date of Web Publication | 18-Apr-2017 |
Correspondence Address:
Moses Bassey Ekong
Department of Anatomy, Faculty of Basic Medical Sciences, University of Uyo, Uyo
Nigeria
 Source of Support: None, Conflict of Interest: None  | Check |
DOI: 10.4103/2315-7992.204682

Introduction: Caffeine is a Psychostimulant consumed as natural components in chocolates, coffees and teas, and as added components to soda, energy drinks, and some drugs. It has been reported to impair the brain in several ways that might lead to activity breakdown. Aim: The present study therefore investigated the potency of caffeine on the neurobehavior and histomorphology of the frontal cortex of a murine model. Materials and Methods: Thirty albino mice were divided into five groups (n = 6), administered intraperitoneally 0.2 ml distilled water, 25, 30, 40 and 60 mg/kg body weight (bw) of caffeine, respectively for 14 days, while the bws were measured prior and after the experiment. On day 15, the dark and light field behavioral test was carried out and the animals were sacrificed by, perfusion method, and the frontal cortices excised from whole brains and routinely processed for histological studies. Results: The mice gained bw in the 25 and 30 mg/kg bw caffeine groups, but lost weight in the 40 and 60 mg/kg bw caffeine groups. No difference was observed in the entire light and dark field test parameters, while histological studies showed significant (P < 0.05) hyperplasia of the frontal cortical cells in the caffeine test groups, all compared with the control and among the test groups. Conclusion: Consumption of the given low dose of caffeine, caused gain in weight while high dose of caffeine caused bw loss, but did not affect the dark and light field behavioral parameters, but stimulated frontal cortical cell hyperplasia possibly as a protective measure.
Keywords: Caffeine, frontal cortex, histomorphology, mice, neurobehavior
How to cite this article:
Ekong MB, Akpan EOH, Nwakanma AA. Murine's lateral frontal cortical histomorphology and its behavior after caffeine administration. Ann Bioanthropol 2016;4:84-9 |
| Introduction | |  |
Caffeine is a psychostimulant and mild diuretic consumed through the world as natural components of chocolates, coffees and teas, and as added components to soda and energy drinks.[1],[2] It is also a common ingredient in diet pills and some other drugs.[3] Caffeine is also known as caffeine, theine, mateine, guaranine, methyltheobromine, and is the common name for 1, 3, 7-trimethlyxanthine or 3,7-dihydro-1, 3, 7-trimethyl-1H-purine-2,6-dione, with the formula C8H10N4O2.[4] It is a white crystalline solid with molar mass, 194.19 g/mol, and density, 1.23 g/ml.[5]
Caffeine is rapidly and completely absorbed from the gastrointestinal tract,[6] and is metabolized by the liver to form dimethyl- and mono-methylxanthines, di-methyl and mono-methyl uric acids, tri-methyl- and dimethyl-allantoin, and uracil derivatives.[7] In the brain, it acts through several mechanisms, but majorly by counteracting adenosine which it does by blocking its receptors.[8],[9],[10]
Clinically, caffeine is used for the management of asthma, gall bladder disease, attention deficit-hyperactivity disorder, shortness of breath in newborns, and low blood pressure.[11] It has also been used for treating different forms of migraine, obesity and in the management of diabetes type 2 and seizures.[12],[13],[14],[15] Long-term consumption of caffeine is associated with a lower risk of cardiovascular disease,[16],[17] while it has been reported to increase the metabolic rate of digestion.[18] It is also reported to protect against Parkinson-like features.[19]
Toxicity of caffeine has been reported in different species,[4],[20],[21] and can present as a spectrum of clinical symptoms. Most of these originate in the central nervous and circulatory systems following ingestion of 1g or more of caffeine.[22] These effects include insomnia, restlessness, increased blood pressure, tachycardia, diuresis, gastrointestinal irritation, muscle twitching, irregular or rapid heartbeat, breakdown of skeletal muscle tissues, dehydration and excitement progressing to mild delirium.[20],[23],[24] High caffeine consumption also accelerates bone loss at the spine in elderly postmenopausal women.[25] An acute overdose of caffeine usually in excess of about 300 mg/kg can over-stimulate the central nervous system.[20]
In humans, caffeine acts as a central nervous system stimulant, temporarily warding off drowsiness, and restoring alertness.[12] Caffeine impairs the brain in several ways: and by producing dependence, can cause or worsen psychiatric illnesses,[22] impairs physical and mental performance,[23] interferes with sleep, influence the risk of other illnesses that in turn exert frontal lobe effects through physical or mental stress [22] and can cause toxicity or even death. Caffeine impinges on the brain's communication system by crossing the blood brain-barrier since it is both water- and lipid-soluble.[26]
Although literatures abound on the activity of caffeine in the body and particularly the brain, little is reported on its role in brain morphology. It is against this background that this research was carried out to investigate the effect of caffeine on some behavioral parameters and the histomorphology of the frontal cortex of albino mice.
| Materials and Methods | |  |
Animal handling
Thirty young female albino mice of average weight 25 g were used for the experiment. They were obtained and kept in the Faculty of Basic Medical Science Animal House, University of Uyo and were handled in accordance with International Guidelines for Animal Care and Use. The animals were housed in wooden cages and were fed with standard feed pellets from Vital Feed Company Ltd., Nigeria and clean drinking water. The animals were allowed 12:12 h' light and dark condition and room temperature of 27°C was maintained throughout. The animals were allowed 2 weeks of acclimatization before the beginning of the experiment and were divided into five groups of six animals each.
Caffeine preparation and administration
0.5 g pure caffeine (No. 101187527, Sigma-Aldrich, England) with molecular weight 194.19 g/mol was dissolved in 100 ml of distilled water in a glass jar forming the caffeine stock solution and the dose for administration was calculated. Group 1 animals were used as the control and were administered 0.2 ml of distilled water, intraperitoneally (i.p.), while Groups 2–5 received 25, 30, 40 and 60 mg/kg body weight (bw) of caffeine i.p., respectively for 14 days. On day 15 of the experiment, dark and light field behavioral test was carried out and the animals were sacrificed after chloroform anesthesia.
Briefly, the behavioral test was carried out using the light/dark maze (box). The test is based on the mice innate aversion to brightly illuminated areas and on the spontaneous exploratory behavior of the mice. The box consisted of a small dark compartment (one-third, painted black) and a large illuminated aversive compartment (two-thirds, painted white) with lines at the base dividing each chamber into small squares. Each mouse was placed in the large white chamber of the box and allowed to move freely between the two chambers. The experiment lasted for 5 min. The number of transition between each chambers and the time spent in each chambers, as well as number of fecal boli and urine puddles were observed and recorded.[27] Furthermore, behavioral parameters such as grooming (an act whereby the mouse scratches any or all part of its body) and rearing (an act whereby the mouse stands on its hindlegs), as well as lines crossed (horizontal movement) by each mouse were observed and recorded.
The animals were sacrificed after chloroform anesthesia and perfusion-fixed with 10% buffered formalin. The whole brain was then excised and postfixed in 10% buffered formalin. On complete fixation at 7 days, the frontal cortex was excised for further processing by hematoxylin and eosin staining method. Tissues were viewed under the microscope to visualize the morphological changes of the frontal cortex. Photomicrographs of each slide were obtained with the aid of a computer-assisted digital camera attached to the microscope.
Cellular populations were determined with WCIF ImageJ™ software (version 1.77c, National Institutes of Mental Health, Bathesda, Maryland, USA). Briefly, live images (at the predetermined area) of the sections were captured using the ImageJ™ software through the light microscope at ×100 magnification. They were converted to 8-bit images and threshold to 200 at the scale of 1 μm while ensuring that the scale was in the global mode. Microscopic scale was then set for camera binding of 1 × 1 at ×10 objectives. Nuclei of the cells were then quantified at this magnification.
One-way analysis of variance was applied to analyze data, and post hoc Tukey's test was used to compare individual groups. Charts were used to represent some of the data, while the table was presented as mean ± standard error of mean. Data with probability level P< 0.05 was regarded as statistically significant.
| Results | |  |
Body weight change
There was bw gain in 25 mg/kg bw (0.20, 0.96%) and 30 mg/kg bw (1.00, 4.25%) caffeine groups compared with the control group (0.50, 2.34%), however, there was bw loss in the 40 mg/kg (−0.33, 1.18%) and 60 mg/kg (−0.33, 1.18%) caffeine groups compared with the control group (0.50, 2.34%) [Figure 1].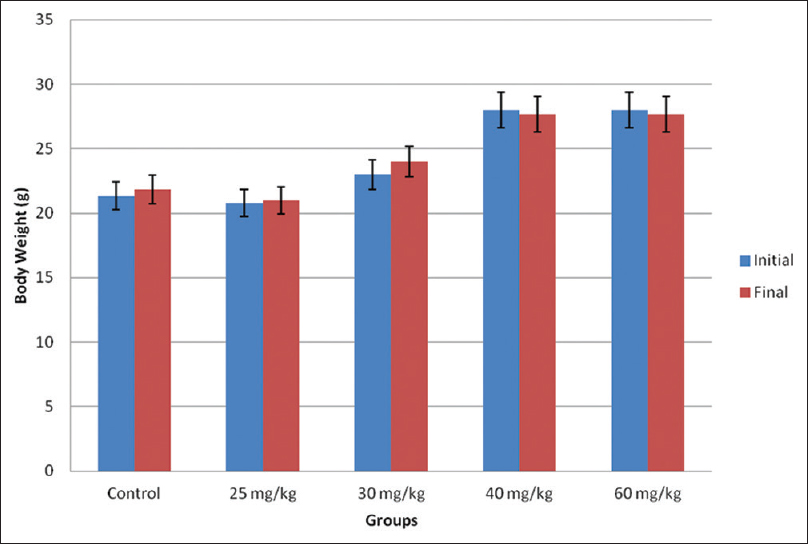 | Figure 1: The body weight change in the experimental animals. Data are presented as mean ± standard error of mean
Click here to view |
Neurobehavioral test
There was no difference in all the light and dark field test parameters; horizontal and vertical movements (line crossing, transition, and rearing frequencies) [Figure 2], grooming frequency, time in the dark box, time in the light box, defecation boli, and urine puddles (none) [Figure 3].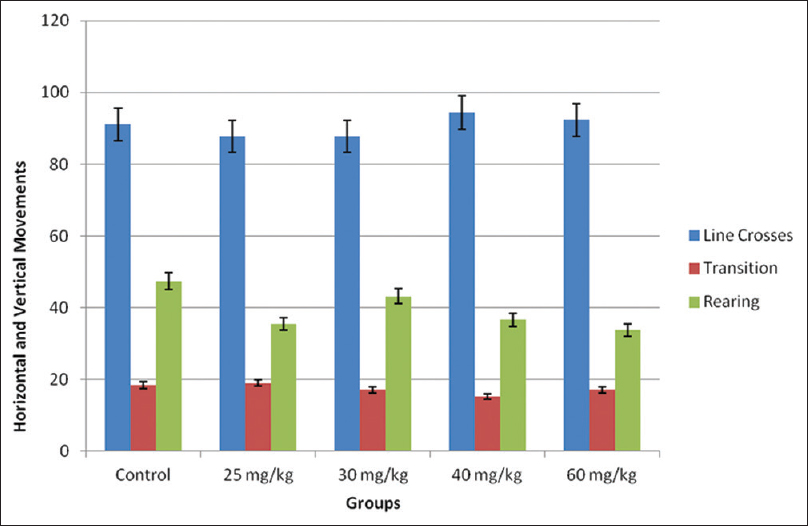 | Figure 2: Horizontal and vertical movements in the light and dark field. No significant difference was recorded compared with the control group at P < 0.05
Click here to view |
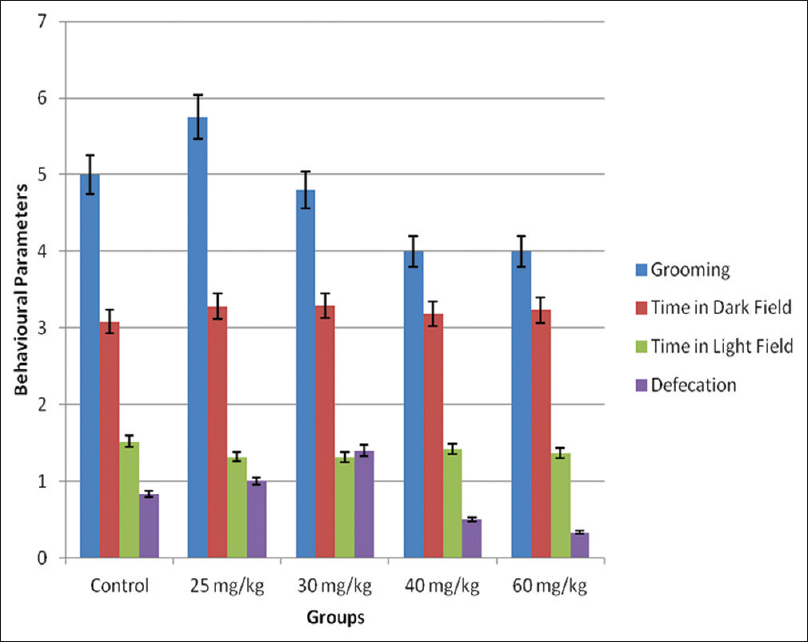 | Figure 3: Other behavioral parameters in the light and dark field. No significant difference was recorded compared with the control group at P < 0.05
Click here to view |
Histomorphology/histomorphometry of the frontal cortex
The histological section of the frontal cortex of the mice in the control group showed six cortical layers, namely, marginal zone, cortical plate, subcortical plate, intermediate, subventricular, and ventricular layers. These are analogues of the six cortical layers in humans. The marginal zone is made up of mostly neuronal fibers and sparse cell density. The cortical plate contained dense cells of different sizes that blended with the subcortical plate. The intermediate and subventricular layers were not easily distinguishable, and contained smaller size cell density [Figure 4]a.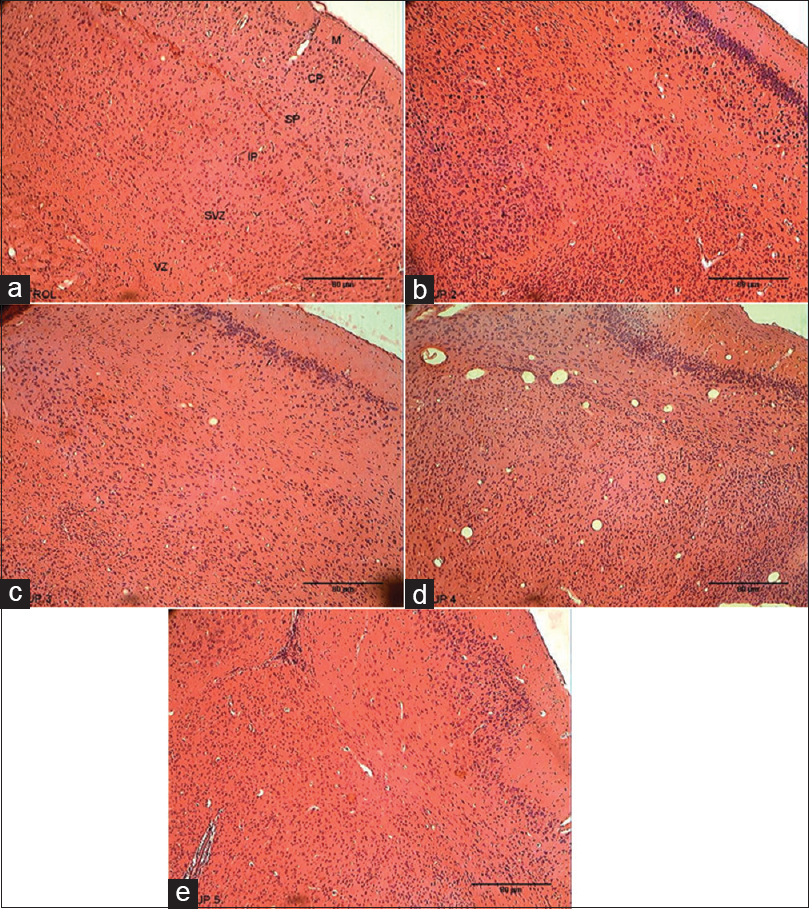 | Figure 4: Photomicrographs of the frontal cortex. (a). Control Group indicating no histopathological changes. (b) 25 mg/kg caffeine group showing increase in cell density in the frontal cortex. (c) 30 mg/kg caffeine group showing increase in cell density in the frontal cortex. (d) 40 mg/kg caffeine group showing increase in cell density in the frontal cortex. (e) 60 mg/kg caffeine showing increase in cell density in the frontal cortex (H and E, ×100). M = Marginal zone; CP = Cortical plate; SP = Subcortical plate; IP = Intermediate plate; SVZ = Subventricular zone; VZ = Ventricular zone
Click here to view |
The histological section of the frontal cortex of the 25 mg/kg caffeine group mice showed high cellular density throughout the cortical layers. The intermediate, subventricular and ventricular layers were not distinguishable compared with the control group [Figure 4]b. The histological section of the frontal cortex of the 30 mg/kg caffeine group mice showed dense cell population of cells, except within the subcortical plate were the cells were less dense compared with the control group [Figure 4]c. The histological section of the frontal cortex of the 40 mg/kg caffeine group mice showed a high cellular density throughout the entire layers of the cortical section, while the subcortical plate, intermediate plate, subventricular, and ventricular layers are less distinguishable compared with the control group [Figure 4]d.
The histological section of the frontal cortex of the 60 mg/kg caffeine group mice showed a high cellular density with smaller size cells. The cortical plate showed a dense aggregation of different cell sizes and types. The subcortical and intermediate plates are less distinguishable with less cellular density. The subventricular and ventricular layers are less distinguishable with high cellular density compared with the control group [Figure 4]e.
There was significant (P < 0.05) increase in cellular population in the frontal cortex in all the Caffeine Groups compared with the Control. The 40 mg/kg caffeine Group had significantly (P < 0.05) higher cellular population compared to the other caffeine groups whereas the 30 mg/kg caffeine Group had a significantly (P < 0.05) lower cellular population compared the 25 mg/kg and 60 mg/kg bw of caffeine [Table 1].
The mean cellular sizes was significantly (P < 0.05) larger in the 25 mg/kg and 40 mg/kg caffeine groups, but lesser in the 60 mg/kg caffeine group compared with the control group. There was no difference in the mean cellular size between the 30 mg/kg caffeine group and the Control. The mean cellular size of the 25 mg/kg caffeine group was significantly (P < 0.05) larger compared with the other caffeine groups, while that of 60 mg/kg caffeine Group was significantly (P < 0.05) smaller compared with the 40 mg/kg caffeine Group [Table 1].
| Discussion | |  |
Caffeine is reported to cause diverse effects in the body. Clinically, this substance is also used for obesity management, and in the management of migraine and diabetes type 2, as well as apnea in neonates.[12],[13],[14],[15] The study was therefore to ascertain the effects of caffeine on some frontal cortical function using the dark and light field behavioral parameters and its histology in a murine model. There were bw gains in the 25 mg/kg and 30 mg/kg caffeine groups, while the 40 mg/kg and 60 mg/kg caffeine groups had bw losses in the course of the experiment. These results indicated that caffeine may stimulate bw gain at a low dose and bw loss at high doses. The bw loss results was in line with previous reports that high caffeine consumption leads to bw loss.[28],[29] A previous report stated that caffeine elicits bw loss through thermogenesis by inhibiting the phosphodiesterase-induced degradation of intracellular cyclic adenosine mono-phosphate.[30] Lee et al.[31] proffered that bw loss was associated with a substantial reduction in insulin-mediated glucose uptake. However, increased bw even with low caffeine consumption had also been reported.[29]
The frontal cortex is often associated with memories associated with emotions derived from inputs from the brain's limbic system,[32] and the dark and light field test is widely used to measure such emotional behavior in rodents. This test is based on the rodents' innate aversion to brightly illuminated areas and on the spontaneous exploratory behavior of the animals.[33],[34] There was no difference in all the measured parameters in the dark and light field test, an indication that the administered caffeine doses and duration was not sufficient to cause a substantial effect. The present behavioral results was at variance with the previous ones.[9],[35] Decreased and increased horizontal and vertical motor activity was reported in CD-1 mice administered caffeine, caffeine analogue, and caffeine-sodium benzoate, respectively.[35] El Yacoubi et al.[36] reported that the stimulant effect of low doses of caffeine is mediated by A2A receptor blockade, while the depressant effect seen at higher doses under some conditions is explained by A1 receptor blockade. This hypothesis may have played a role in the present study.
Histological studies showed hyperplasia of the frontal cortical cells in the Caffeine Test Groups. Caffeine has been reported to cross the blood-brain barrier,[4] and the present results may suggest a stimulation of either gliosis or/and neurogenesis. Gliosis usually occurs when there is the presence of chemical agents, such as caffeine in the brain. It is reported that caffeine stimulates gliosis in the striatum, hippocampus, substantia nigra pars compacta and cerebral cortex,[19],[37] and this gliosis may be the resulting effect as seen in the cellular hyperplasia of the histological study. Neurogenesis has been reported to occur in the frontal cortex.[38] However, reports show that caffeine inhibits neurulation and adult neurogenesis.[39],[40],[41] Hence, neurogenesis may be ruled out in the present study.
The frontal cortex contains most of the dopamine-sensitive neurons in the cerebral cortex,[42] and plays an important part in retaining long term memories which are not task-based, and are often associated with emotions derived from inputs from the brain's limbic system.[42] The frontal cortex therefore modifies these emotions to generally fit socially acceptable norms.[43],[44] This dopamine system is also associated with reward, attention, short-term memory tasks, planning and motivation.[45] Caffeine has been reported to improve these functions,[46] but this may not be through neurogenesis.
Caffeine use has been linked with specific disorders such as anxiety disorders, sleep disorders and eating disorders, and there is a possible association with schizophrenia. Sensitivity to caffeine is increased in people with panic disorder and social phobia, and administration of caffeine can provoke panic attacks in these individuals.[27] As caffeine caused hyperplasia of frontal cortical cells with the target mostly likely the glia, it will lead to increase nutrients and protection to the brain that may ultimately be injurious on the long run.
| Conclusion | |  |
Consumption of the given low and high doses of caffeine caused bw increase and loss respectively, but both low and high doses did not affect the dark and light field behavioral parameters, but stimulated frontal cortical cell hyperplasia possibly as a protective measure.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| References | |  |
| 1. | Wanyika HN, Gatebe EG, Gitu LM, Ngumba EK, Maritim CW. Determination of caffeine content of tea and instant coffee brands found in the Kenyan market. Afr J Food Sci 2010;4:353-8.  |
| 2. | Seifert SM, Schaechter JL, Hershorin ER, Lipshultz SE. Health effects of energy drinks on children, adolescents, and young adults. Pediatrics 2011;127:511-28.  |
| 3. | Derry CJ, Derry S, Moore RA. Caffeine as an analgesic adjuvant for acute pain in adults. Cochrane Database Syst Rev 2012;14(3):CD009281.  |
| 4. | Peters JM. Factors affecting caffeine toxicity: A review of the literature. J Clin Pharmacol J New Drugs 1967;7:131-41.  |
| 5. | Chen QC, Wang J. Simultaneous determination of artificial sweeteners, preservatives, caffeine, theobromine and theophylline in food and pharmaceutical preparations by ion chromatography. J Chromatogr A 2001;937:57-64.  |
| 6. | Magkos F, Kavouras SA. Caffeine use in sports, pharmacokinetics in man, and cellular mechanisms of action. Crit Rev Food Sci Nutr 2005;45:535-62.  |
| 7. | Arnaud MJ. Metabolism of caffeine and other components of coffee. In: Garattini S, editor. Caffeine, Coffee and Health. New York: Raven Press; 1993. p. 43-95.  |
| 8. | Choi OH, Shamim MT, Padgett WL, Daly JW. Caffeine and theophylline analogues: Correlation of behavioral effects with activity as adenosine receptor antagonists and as phosphodiesterase inhibitors. Life Sci 1988;43:387-98.  |
| 9. | Kaplan GB, Greenblatt DJ, Kent MA, Cotreau-Bibbo MM. Caffeine treatment and withdrawal in mice: Relationships between dosage, concentrations, locomotor activity and A1 adenosine receptor binding. J Pharmacol Exp Ther 1993;266:1563-72.  |
| 10. | Fredholm BB. Astra award lecture. Adenosine, adenosine receptors and the actions of caffeine. Pharmacol Toxicol 1995;76:93-101.  |
| 11. | Jahanfar S, Sharifah H. Effects of restricted caffeine intake by mother on fetal, neonatal and pregnancy outcome. Cochrane Database Syst Rev 2009;15(2):CD006965.  |
| 12. | Bruce M, Scott N, Shine P, Lader M. Anxiogenic effects of caffeine in patients with anxiety disorders. Arch Gen Psychiatry 1992;49:867-9.  |
| 13. | Goldstein J, Silberstein SD, Saper JR, Ryan RE Jr., Lipton RB. Acetaminophen, aspirin, and caffeine in combination versus ibuprofen for acute migraine: Results from a multicenter, double-blind, randomized, parallel-group, single-dose, placebo-controlled study. Headache 2006;46:444-53.  |
| 14. | Gilmore B, Michael M. Treatment of acute migraine headache. Am Fam Physician 2011;83:271-80.  |
| 15. | Dobson NR, Hunt CE. Pharmacology review: Caffeine use in neonates: Indications, pharmacokinetics, clinical effects, outcomes. Neoreviews 2013;14:e540-50.  |
| 16. | Thompson R, Keene K. The pros and cons of caffeine. Psychologist 2004;17:698-701.  |
| 17. | van Dam RM. Coffee consumption and risk of type 2 diabetes, cardiovascular diseases, and cancer. Appl Physiol Nutr Metab 2008;33:1269-83.  |
| 18. | Koot P, Deurenberg P. Comparison of changes in energy expenditure and body temperatures after caffeine consumption. Ann Nutr Metab 1995;39:135-42.  |
| 19. | Chen X, Lan X, Roche I, Liu R, Geiger JD. Caffeine protects against MPTP-induced blood-brain barrier dysfunction in mouse striatum. J Neurochem 2008;107:1147-57.  |
| 20. | Benowitz NL. Clinical pharmacology of caffeine. Annu Rev Med 1990;41:277-88.  |
| 21. | Klosterman L. The Facts about Caffeine (Drugs). New York: Benchmark Books; 2006. p. 43.  |
| 22. | Synder SH. Adenosine receptors and the actions of methylxanthines. Trends Neurosci 1981;4:242-4.  |
| 23. | Johnson-Kozlow M, Kritz-Silverstein D, Barrett-Connor E, Morton D. Coffee consumption and cognitive function among older adults. Am J Epidemiol 2002;156:842-50.  |
| 24. | Smith A. Effects of caffeine on human behavior. Food Chem Toxicol 2002;40:1243-55.  |
| 25. | Rapuri PB, Gallagher JC, Kinyamu HK, Ryschon KL. Caffeine intake increases the rate of bone loss in elderly women and interacts with Vitamin D receptor genotypes. Am J Clin Nutr 2001;74:694-700.  |
| 26. | Kimberg DY, Farah MJ. A unified account of cognitive impairments following frontal lobe damage: The role of working memory in complex, organized behavior. J Exp Psychol Gen 1993;122:411-28.  |
| 27. | Lister RG. Ethologically-based animal models of anxiety disorders. Pharmacol Ther 1990;46:321-40.  |
| 28. | Kovacs EM, Lejeune MP, Nijs I, Westerterp-Plantenga MS. Effects of green tea on weight maintenance after body-weight loss. Br J Nutr 2004;91:431-7.  |
| 29. | Westerterp-Plantenga MS, Lejeune MP, Kovacs EM. Body weight loss and weight maintenance in relation to habitual caffeine intake and green tea supplementation. Obes Res 2005;13:1195-204.  |
| 30. | Dulloo AG. Ephedrine, xanthines and prostaglandin-inhibitors: Actions and interactions in the stimulation of thermogenesis. Int J Obes Relat Metab Disord 1993;17 Suppl 1:S35-40.  |
| 31. | Lee S, Hudson R, Kilpatrick K, Graham TE, Ross R. Caffeine ingestion is associated with reductions in glucose uptake independent of obesity and type 2 diabetes before and after exercise training. Diabetes Care 2005;28:566-72.  |
| 32. | Durani SK, Ford R, Sajjad SH. Capgras syndrome associated with a frontal lobe tumour. Ir J Psychol Med 1991;8:135-6.  |
| 33. | Costall B, Jones BJ, Kelly ME, Naylor RJ, Tomkins DM. Exploration of mice in a black and white test box: Validation as a model of anxiety. Pharmacol Biochem Behav 1989;32:777-85.  |
| 34. | Bourin M, Hascoët M. The mouse light/dark box test. Eur J Pharmacol 2003;463:55-65.  |
| 35. | Kaplan GB, Greenblatt DJ, Kent MA, Cotreau MM, Arcelin G, Shader RI. Caffeine-induced behavioral stimulation is dose-dependent and associated with A1 adenosine receptor occupancy. Neuropsychopharmacology 1992;6:145-53.  |
| 36. | El Yacoubi M, Ledent C, Ménard JF, Parmentier M, Costentin J, Vaugeois JM. The stimulant effects of caffeine on locomotor behaviour in mice are mediated through its blockade of adenosine A (2A) receptors. Br J Pharmacol 2000;129:1465-73.  |
| 37. | Khairnar A, Plumitallo A, Frau L, Schintu N, Morelli M. Caffeine enhances astroglia and microglia reactivity induced by 3,4-methylenedioxymethamphetamine ('ecstasy') in mouse brain. Neurotox Res 2010;17:435-9.  |
| 38. | Jiang W, Gu W, Brännströ m T, Rosqvist R, Wester P. Cortical neurogenesis in adult rats after transient middle cerebral artery occlusion. Stroke 2001;32:1201-7.  |
| 39. | Marret S, Gressens P, Van-Maele-Fabry G, Picard J, Evrard P. Caffeine-induced disturbances of early neurogenesis in whole mouse embryo cultures. Brain Res 1997;773:213-6.  |
| 40. | Han ME, Park KH, Baek SY, Kim BS, Kim JB, Kim HJ, et al. Inhibitory effects of caffeine on hippocampal neurogenesis and function. Biochem Biophys Res Commun 2007;356:976-80.  |
| 41. | Wentz CT, Magavi SS. Caffeine alters proliferation of neuronal precursors in the adult hippocampus. Neuropharmacology 2009;56:994-1000.  |
| 42. | Semendeferi K, Lu A, Schenker N, Damasio H. Humans and great apes share a large frontal cortex. Nat Neurosci 2002;5:272-6.  |
| 43. | Coffey CE, Wilkinson WE, Parashos IA, Soady SA, Sullivan RJ, Patterson LJ, et al. Quantitative cerebral anatomy of the aging human brain: A cross-sectional study using magnetic resonance imaging. Neurology 1992;42 (3 Pt 1):527-36.  |
| 44. | Stuss DT, Gow CA, Hetherington CR. “No longer Gage”: Frontal lobe dysfunction and emotional changes. J Consult Clin Psychol 1992;60:349-59.  |
| 45. | Rowe AD, Bullock PR, Polkey CE, Morris RG. “Theory of mind” impairments and their relationship to executive functioning following frontal lobe excisions. Brain 2001;124(Pt 3):600-16.  |
| 46. | Higashi T, Sone Y, Ogawa K, Kitamura YT, Saiki K, Sagawa S, et al. Changes in regional cerebral blood volume in frontal cortex during mental work with and without caffeine intake: Functional monitoring using near-infrared spectroscopy. J Biomed Opt 2004;9:788-93.  |
[Figure 1], [Figure 2], [Figure 3], [Figure 4]
[Table 1]
|