|
 
 |
|
ORIGINAL ARTICLE |
|
|
|
| Year : 2011 | Volume
: 17
| Issue : 2 | Page : 65-69 |
| |
Molecular investigation of mental retardation locus gene PRSS12 by linkage analysis
Zafar Ali, Masroor Ellahi Babar, Jamil Ahmad, Muhammad Zubair Yousaf, Muhammad Asif, Sajjad Ali Shah
Department of Biotechnology and Informatics, BUITEMS, Quetta, Pakistan, Institute of Biochemistry and Biotechnology, University of Veterinary and Animal Sciences, Lahore, Pakistan
| Date of Web Publication | 17-Oct-2011 |
Correspondence Address:
Masroor Ellahi Babar
Institute of Biochemistry and Biotechnology, University of Veterinary and Animal Sciences, Lahore
Pakistan
 Source of Support: None, Conflict of Interest: None
DOI: 10.4103/0971-6866.86178

 Abstract Abstract | | |
The present study was carried out to determine the prevalence of families having mental retardation in Pakistani population. We enrolled seven mentally retarded (MR) families with two or more affected individuals. Family history was taken to minimize the chances of other abnormalities. Pedigrees were drawn using the Cyrillic software (version 2.1). The structure of pedigrees shows that all the marriages are consanguineous and the families have recessive mode of inheritance. All the families were studied by linkage analysis to mental retardation locus (MRT1)/gene PRSS12. Three STR markers (D4S191, D4S2392, and D4S3024) in vicinity of mental retardation (MR) locus (MRT1)/gene PRSS12 were amplified on all the sample of each family by PCR. The PCR products were then genotyped on non denaturing polyacrylamide gel electrophoresis (PAGE). The Haplotype were constructed to determine the pattern of inheritance and also to determine that a family was linked or unlinked to gene PRSS12. One out of the seven families was potentially linked to gene PRSS12, while the other six families remain unlinked.
Keywords: Linkage analysis, mental retardation, mental retardation locus, neurotrypsin, PRSS12
How to cite this article:
Ali Z, Babar ME, Ahmad J, Yousaf MZ, Asif M, Shah SA. Molecular investigation of mental retardation locus gene PRSS12 by linkage analysis. Indian J Hum Genet 2011;17:65-9 |
How to cite this URL:
Ali Z, Babar ME, Ahmad J, Yousaf MZ, Asif M, Shah SA. Molecular investigation of mental retardation locus gene PRSS12 by linkage analysis. Indian J Hum Genet [serial online] 2011 [cited 2016 May 13];17:65-9. Available from: http://www.ijhg.com/text.asp?2011/17/2/65/86178 |
 Introduction Introduction | |  |
Mental retardation (MR) can be defined as the failure to develop a sufficient cognitive and adaptive level. It is one of the most common human disorders. MR is associated with functional deficit in adaptive behaviour, such as daily-living skills, social skills, and communication. [1] It is either the only consistent handicap (called nonsyndromic MR) or is combined with other physical and/or behavioural abnormalities (called syndromic MR). [2],[3]
MR or developmental delay is a heterogeneous group of disorders. The prevalence of MR is commonly given as 1%-3% of the population, with a proportion higher in males than females (1.4:1). [4] MR is sub classified according to intelligence quotient (IQ) as mild MR in the IQ range 50-70, moderate MR as 35-55, severe MR as 20-40, and profound MR as below 20-25. [4] It can also be classified as, nonsyndromic and syndromic. The nonsyndromic is further divided into autosomal MR and X-linked MR then further autosomal MR is divided into autosomal recessive non syndromic mental retadation (ARNSMR) and autosomal dominant.
The causes of nearly 40% of MR remain unclear. Although a considerable number of X-linked MR (XLMR) genes are known, only four genes causing autosomal recessive nonsyndromic MR (ARNSMR) have been identified so far: PRSS12, CRBN, CC2D1A, and GRIK2. These genes are involved in different pathways, namely synaptic proteolysis, regulation of mitochondrial energy metabolism, regulation of I-kB kinase/NF-kB cascade and induction of long-term potentiation (LTP), underlining the extreme heterogeneity of the pathophysiological mechanism involved in these diseases. [5]
Neurotrypsin have various roles in the central nervous system both physiological and pathological. [6],[7],[8],[9] Two groups [10],[11] identified neurotrypsin independently and simultaneously. The names neurotrypsin, PRSS12 and motopsin were given by HUGO nomenclature. It is secreted from neuronal cells in brain regions like hippocampus, the cerebral cortex, and the cranial nerve nuclei. [10],[12] In the hippocampus the neurotrypsin mRNA is expressed most abundantly during the first postnatal week, which then gradually decreases but still continues into adult life. [12],[13] During the perinatal period, the abundant expression of neurotrypsin mRNA is observed in other regions like olfactory system, cranial nerve nuclei, spinal cord and peripheral nervous system, which shows that neurotrypsin performs many roles in the development of nervous system. [14]
Recessively inherited diseases are more prevalent in populations where cousin marriages are common, like Pakistan. [15] These large consanguineous families are a powerful resource for genetic linkage studies of recessively inherited disorders like mental retardation. Linkage analysis was the major objective of this study and it may play a role in creating awareness about the effect of cousin marriages that is the first step towards decreasing socio-economic burden of the country by genetic counseling and also to prevent mental retardation in Pakistan due to inbreeding.
 Materials and Methods Materials and Methods | |  |
Enrollment of the families
Families with at least two or more individual affected with mental retardation were selected from different areas of District Swat and District Peshawar of Khyber Pakhtunkhwa Province of Pakistan. Detailed family history was taken to minimize the chances of other abnormalities and pedigree was made personally by visiting each family. The pedigrees were made by using the Cyrillic software. Relatives of the family affected with MR were also included in the study depending on their willingness and availability. Informed consent was also obtained for participating in the study.
Collection of blood samples
Blood samples (5 mL) were collected from all the affected individuals, their normal siblings, parents to trace the mode of inheritance. The blood samples were collected in 50 mL falcon tubes already containing 140 ΅l Ethylene Diamine Tetra Acetate (EDTA), which work as an anticoagulant, the blood samples were stored in ice boxes immediately after their collection and then the blood samples were stored in freezer before DNA extraction.
Deoxyribonucleic Acid extraction
Genomic DNA was extracted from blood samples by inorganic method. [16] This method consists of three main steps, (1) lysis of RBCs with the T.E lysis buffer (Tris HCl, EDTA), which was done by washing the blood two or three times with T.E lysis buffer. (2) lysis of WBCs and digestion of proteins with 100 mL of 10% SDS, 40 mL proteinase K and 3 mL TNE Buffer (Tris HCL, EDTA, and NaCl) by adding to the pellet, and incubated for 12-18 hours in shaking water bath at 45 o C for complete digestion of cellular proteins and WBCs. (3) salting out of proteins by adding 1 mL of 6 M NaCl to the digested samples after the period of incubation. In this way, all the proteins were salted out leaving behind the supernatant containing the DNA. It was then precipitated by adding equal volume of isopropanol and DNA was obtained in the form of pellet by centrifugation.
Deoxyribonucleic Acid quantification
The DNA quantification was done on 0.8% agarose gel.
Genotyping for mental retardation locus
For MRT1 locus, three STR markers were typed. Genotyping was performed with unlabeled primers. The markers used for linkage analysis encompassed the chromosomal locations as mentioned by UCSC Genome Browser. For genotyping of the DNA samples of a particular family, each marker was amplified separately on each DNA samples of all seven families. Primers were optimized for their annealing temperatures and recipe for Polymerase Chain Reaction (PCR). All the primers were amplified by a touch down PCR in which a range of annealing temperature (50°C-60°C) was used. Amplification reactions were carried out at temperatures and recipe specific for each STR marker (primer).
Polyacralamide gel electrophoresis
Polyacralamide gel electrophoresis products of all the STR marker of all the seven families were run on non denaturing to examine amplified bands of the STR marker for linkage analysis and to determine the mode of inheritance. [17]
 Results Results | |  |
A total of seven families with two or more affected individuals were identified from the different areas of District Swat and District Peshawar and were enrolled in the present study. The blood samples were collected from these families and DNA was extracted by standard inorganic protocol.
After DNA extraction, linkage analysis studies were carried out. Three STR markers D4S191, D4S2392, and D4S3024 were genotyped to determine if a family was linked or unlinked to mental retardation locus (MRT1)/gene PRSS12 [Table 1]. Hapalotypes (set of alleles) were constructed to determine the pattern of inheritance among the affected and normal individuals of each family under study.
One out of the seven families was potentially linked to mental retardation locus (MRT1)/gene PRSS12. All the other six families remained unlinked to mental retardation locus (MRT1)/gene PRSS12 [Table 2]. | Table 2: Status of pedigrees analyzed by Linkage analysis for MRT1 Gene Prss12
Click here to view |
Family mental retardation-05
The family MR-05 was collected from village swegale tehsil kabal, district Swat, Pakistan. The entire affected individuals were appearing in the fourth generation. Blood samples of 5 individuals were collected, having two affected individual (IV:1 and IV:2) along with one normal (IV:3) and their mother (III:3) along with their uncle (III: 1) in this family. The affected individuals range in age from 11 to 14 years. After DNA isolation, three STR markers D4S191, D4S2392, and D4S3024 spanning in the region of MRT1 gene PRSS12 were amplified. PCR product was electrophoresed on 7% Polyacrylamide gel at 150 volts for 2 to 3 hours. Polyacrylamide gel was stained with ethedium bromide. Alleles were read manually with larger allele donated by 2 and smaller with 1. Haplotypes of family MR-05 showed potential linkage to MRT1 gene PRSS12. All individuals (both affected and normal) were homozygous with STR marker D4S2392 and D4S191 except their mother which was heterozygous with both the markers and with STR marker D4S3024 all individuals were homozygous except their uncle who was heterozygous at MRT1 gene PRSS12 [Figure 1].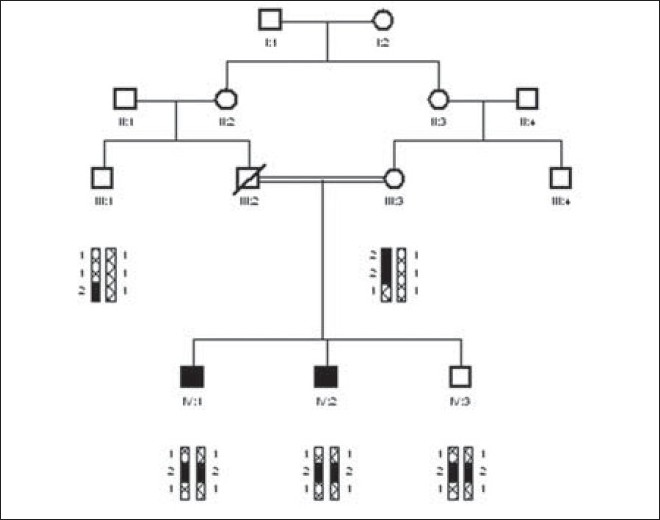 | Figure 1: Pedigree of family MR-05. The three STR markers D4S191, D4S2392, and D4S3024 in candidate region of mental retardation locus MRT1 gene PRSS12 showed potential linkage. Mental retardation phenotype in this family was potentially linked to mental retardation locus MRT1 gene PRSS12
Click here to view |
 Discussion Discussion | |  |
MR is a complex phenotype characterized by suboptimal functioning of the central nervous system resulting in significant limitations both in intellectual functioning and in adaptive behavior as expressed in conceptual, social and practical adaptive skills originating before 18 years of age. [18] MR is one of the most common genetic disorders with an estimated incidence of 1-3% of the general population with a high proportion of males to females (1.4:1). [4]
It can be classified as either syndromic or nonsyndromic. In patients with syndromic forms of MR, the clinical manifestations include MR associated with other multiple congenital anomalies and defects in organs/tissues other then brain and other physical features (such as neurological symptoms, skeletal defects or facial dysmorphism), whereas in patients with nonsyndromic MR no distinctive clinical or biochemical manifestations occur, apart from the cognitive impairment. [19] Etiologically, it is a multifactorial disorder manifested by environmental as well as genetic factors. Genetic factors include (chromosomal abnormalities, submicroscopic deletions, and monogenic diseases). [20] Genetic etiologies are found in approximately two thirds of cases of MR. An autosomal recessive mode of inheritance may account for nearly a quarter of all individuals with non-syndromic mental retardation (NSMR). While various X-linked are also associated with non-syndromic mental retardation (NSMR). [21] More than 60 loci have been reported for X-linked MR; however, still the molecular genetic basis for ARMR is poorly understood. [4]
Neurotrypsin (PRSS12) is an extracellular serine proteases and it have various physiological and pathological roles in the central nervous system. [6-9] It was identified by two groups. [10],[11] All the three names PRSS12, neurotrypsin, and motopsin were approved by the HUGO nomenclature. Neurotrypsin is secretion of neuronal cells in various brain regions, like hippocampus, cerebral cortex, and the cranial nerve nuclei. [10],[12] The mRNA of neurotrypsin is expressed most abundantly during the first postnatal week in the hippocampus and the cingulated cortex but its expression gradually decreases and still continues into adult life. [12],[13] The abundant expression of neurotrypsin mRNA During the perinatal period is observed in other regions as well, like olfactory system, cranial nerve nuclei, spinal cord, and peripheral nervous system, which suggest that neurotrypsin plays multiple roles in the developing nervous system. [14]
As a consequence of the unique socio-cultural practices in the population of Pakistan, approximately 60% of marriages are consanguineous, of which more than 80% are between first cousins. [22] Recessively inherited diseases are more prevalent in populations where cousin marriages are common, like Pakistan. [15] These large consanguineous families are a powerful resource for genetic linkage studies of recessively inherited disorders like mental retardation.
In this study, seven families were selected for linkage analysis to MRT1 gene PRSS12. These families were collected from different areas of District Swat and District Peshawar. The families have at least three (except family MR-05 which have two) affected individuals. All the families have recessive mode of inheritance. One out of seven families was potentially linked to MRT1 gene PRSS12, while the other six remain unlinked.
The reason that the families which remain unlinked to the reported loci during screening signifies extreme genetic heterogeneity of MR, which is not surprising because about 50% of human protein coding genes are expressed in the brain. [23] Apart from this there are more than 900 genetic disorders associated with MR and it affects around 3% of the general population, which also reflects the extreme complexity and heterogeneity of mental retardation. [24] Despite recent advances, the causes of nearly 40% of MR remain unclear. Although a considerable number of X-linked MR (XLMR) genes are known, only four genes causing autosomal- recessive nonsyndromic MR (AR-NSMR) have been identified so far: PRSS12, CRBN, CC2D1A, and GRIK2. [5] Only a very few families with ARMR have been confirmed for each of these genes (PRSS12, n = 2; CRBN, n = 1; CC2D1A, n = 9; GRIK2, n = 1). [4] Mutations in known ARNSMR genes have been detected so far in only a small number of families. The contributions of autosomal recessive nonsyndromic mental retardation (ARNSMR) genes are vary limited in the general population. [25]
All the above reason shows that mental retardation is a complex nervous disorder and these families may be linked to already known locus or there are chances that these families may linked to a new loci. So it needs further studies to identify the already known locus or to explore novel loci and gene causing mental retardation in this population. This will provide opportunities of genetic counseling to these populations and will ultimately result in prevention of mental retardation in Pakistani population.
 References References | |  |
| 1. | Chelly J, Khelfaoui M, Francis F, Cherif B, Bienvenu T. Genetics and pathophysiology of mental retardation. Eur J Hum Genet 2006;14:701-13. 
|
| 2. | Curry CJ, Stevenson RE, Aughton D, Byrne J, Carey JC, Cassidy S, et al. Evaluation of mental retardation: Recommendations of a consensus conference. Am J Med Genet 1997;72:468-77. 
|
| 3. | Roeleveld N, Zielhuis GA, Gabreels F. The prevalence of mental retardation: A critical review of recent literature. Dev Med Child Neurol 1997;39:125-32. 
|
| 4. | Noor A, Windpassinger C, Patel M, Stachowiak B, Mikhailov A, Azam M, et al. CC2D2A encoding a coiled-coil and C2 domain protein, causes autosomal-recessive mental retardation with retinitis pigmentosa. Am J Hum Genet 2008;82:1-8. 
|
| 5. | Molinari F, Foulquier F, Tarpey PS, Morelle W, Boissel S, Teague J, et al. Oligosaccharyltransferase-subunit mutations in nonsyndromic mental retardation. Am J Hum Genet 2008;82:1150-7. 
|
| 6. | Yoshida S, Shiosaka S. Plasticity related serine proteases in the brain. Int J Mol Med 1999;3:405-6. 
|
| 7. | Shiosaka S, Yoshida S. Synaptic microenvironments structural plasticity, adhesion molecules, proteases and their inhibitors. Neurosci Res 2000;37:85-9. 
|
| 8. | Tomimatsu Y, Idemoto S, Moriguchi S, Watanabe S, Nakanishi H. Proteases involved in long-term potentiation. Life Sci 2002;72:355-61. 
|
| 9. | Mitsui S, Watanabe Y, Yamaguchi T, Yamaguchi N. Mosaic serine proteases in the mammalian central nervous system. Front Biosci 2008;13:1991-2000. 
|
| 10. | Gschwend TP, Krueger SR, Kozlov SV, Wolfer DP, Sonderegger P. Neurotrypsin, a novel multidomain serine protease expressed in the nervous system. Mol Cell Neurosci 1997;9:207-19. 
|
| 11. | Yamamura Y, Yamashiro K, Tsuruoka N, Nakazato H, Tsujimura A, Yamaguchi N. Molecular cloning of a novel brain-specific serine protease with a kringle-like structure and three scavenger receptor cysteinerich motifs. Biochem Biophys Res Commun 1997;239:386-92. 
|
| 12. | Iijima N, Tanaka M, Mitsui S, Yamamura Y, Yamaguchi N, Ibata Y. Expression of a serine protease (motopsin PRSS12) mRNA in the mouse brain: In situ hybridization histochemical study. Brain Res Mol Brain Res 1999;66:141-9. 
|
| 13. | Wolfer DP, Lang R, Cinelli P, Madani R, Sonderegger P. Multiple roles of neurotrypsin in tissue morphogenesis and nervous system development suggested by the mRNA expression pattern. Mol Cell Neurosci 2001;18:407-33. 
|
| 14. | Mitsui S, Osako Y, Yokoi F, Dang MT, Yuri K, Li Y, et al. A mental retardation gene, motopsin⁄neurotrypsin⁄prss12, modulates hippocampal function and social interaction. Eur J Neurosci 2009;30:2368-78. 
|
| 15. | Jaber L, Halpem GJ, Shohat M. The impact of consanguinity worldwide. Community Genet 1998;1:12-17. 
|
| 16. | Sambrook J, Russell DW. Molecular Cloning, A Laboratory Manual. 3 rd ed. Cold Spring Harbor, New York, USA: Cold Spring Harbor Laboratory Press; 2001. 
|
| 17. | Wang D, Shi J, Carlson SR, Cregan PB, Ward RW, et al. A low-cost, high-throughput polyacrylamide gel electrophoresis system for genotyping with microsatellite DNA markers. Crop Sci 2003;43:1828-32. 
|
| 18. | Chiurazzi P, Schwartz CE, Gecz J, Neri G. XLMR genes: Update 2007. Eur J Hum Genet 2008;16:422-34. 
|
| 19. | Frints SG, Marynen P, Hartmann D, Fryns J, Steyaert J, Schachner M, et al. CALL interrupted in a patient with non-specific mental retardation: Gene dosage dependent alteration of murine brain development and behavior. Hum Mol Genet 2003;12 Suppl 13:1463-74. 
|
| 20. | Ropers HH. Genetics of intellectual disability. Curr Opin Genet Dev 2008;18:241-50. 
|
| 21. | Basel-Vanagaite L, Attia R, Yahav M, Ferland RJ, Anteki L, Walsh CA, et al. The CC2D1A, a member of a new gene family with C2 domains, is involved in autosomal recessive nonsyndromic mental retardation. J Med Genet 2006;43:203-10. 
|
| 22. | Hussain R, Bittles AH. The prevalence and demographic characteristics of consanguineous marriages in Pakistan. J Biosoc Sci 1998;30:261-75. 
|
| 23. | Inlow JK, Restifo LL. Molecular and comparative genetics of mental retardation. Genetics 2004;166:835-81. 
|
| 24. | Castellvi-Bel S, Mila M. Genes responsible for nonspecific mental retardation. Mol Genet Metab 2001;72:104-8. 
|
| 25. | Basel-Vanagaite L. Genetics of autosomal recessive non-syndromic mental retardation: Recent advances. Clin Genet 2007;72:167-74. 
|
[Figure 1]
[Table 1], [Table 2]
|






