|
 
 |
|
ORIGINAL ARTICLE |
|
|
|
| Year : 2011 | Volume
: 17
| Issue : 2 | Page : 77-81 |
| |
Cytoprotective effect of honey against chromosomal breakage in fanconi anemia patients in vitro
Faeza Abdel Mogib El-Dahtory1, Sohier Yahia2
1 Genetic Unit of Children Hospital, Mansoura University, Mansoura, Egypt
2 Department of Pediatrics and Genetics, Faculty of Medicine, Mansoura University, Mansoura, Egypt
| Date of Web Publication | 17-Oct-2011 |
Correspondence Address:
Faeza Abdel Mogib El-Dahtory
Genetic Unit of Children Hospital, Mansoura University, Mansoura
Egypt
 Source of Support: None, Conflict of Interest: None
DOI: 10.4103/0971-6866.86184

 Abstract Abstract | | |
Background : Natural honey is widely used all over the world as a complementary and alternative medicine in various disorders including Fanconi anemia (FA). FA is a rare genetic chromosomal instability syndrome caused by impairment of DNA repair and reactive oxygen species (ROS) imbalance. This disease is also related to bone marrow failure and cancer. The aim of this study was to evaluate the cytoprotective effect of honey on mitomycin C (MMC-) induced chromosomal damage in peripheral lymphocytes from FA patients.
Materials and Methods :Treatment of these complications with alkylation agents MMC may enhance chromosomal breakage. We have evaluated the effect of honey on MMC- induced chromosomal breakage in FA blood cells using chromosomal breakage assay. The basal chromosomal breakage count was higher among FA patients than healthy subjects.
Results : The addition of MMC alone gave a significantly higher of chromosomal breakage in FA patients than control group (P < 0.0001). Pre- treatment with honey significantly inhibited breakage induced by MMC in FA patients by its antioxidant effect.
Conclusion : Honey can prevent MMC- induced chromosomal breakage by its antioxidant effect.
Keywords: Chromosomal breakage, Fanconi anemia, hony, mitomycin-C
How to cite this article:
Mogib El-Dahtory FA, Yahia S. Cytoprotective effect of honey against chromosomal breakage in fanconi anemia patients in vitro. Indian J Hum Genet 2011;17:77-81 |
How to cite this URL:
Mogib El-Dahtory FA, Yahia S. Cytoprotective effect of honey against chromosomal breakage in fanconi anemia patients in vitro. Indian J Hum Genet [serial online] 2011 [cited 2016 May 13];17:77-81. Available from: http://www.ijhg.com/text.asp?2011/17/2/77/86184 |
 Introduction Introduction | |  |
Fanconi anemia is an autosomal recessive chromosomal instability syndrome characterized clinically by developmental abnormalities, growth retardation, progressive bone marrow failure, pancytopenia, and pronounced cancer predisposition. [1],[2] The exact pathogenesis has not been elucidated until now. [3],[4] A striking cellular feature is sensitivity to DNA cross- linking agents, such as MMC and diepoxybutane (DEB). [1],[2] Indeed, elevated chromosome instability following exposure to these agents is used to aid FA diagnosis. [5] Androgen therapy can improve the outcome, [6] but side- effects, such as hepatocarcinoma and tolerance problems arise, [7] thus, bone marrow transplantation has become the only well established treatment for the pancytopenia associated with FA. [6] As addition of superoxide dismutase or catalase to FA cultured cells reduced the frequency of chromosomal aberrations. Later, Joenje et al. found that chromosomal breakage in FA cells augmented with increasing oxygen tension, thus, it was proposed that the primary defect results from a failure to tolerate oxidative stress. [8] On the other hand, antioxidants should help to counter the deleterious effects induced by oxidative stress. Such is the case for mytomicin C (MMC) associated clastogenicity which depends on oxygen levels, [9] as this damage decreases upon addition of low molecular weight antioxidants. [10]
Research indicates that honey contains numerous phenolic and nonphenolic antioxidants. [11] Darker honeys are generally higher in antioxidant content than lighter honeys. [11],[12],[13],[14] Honey has a great potential to serve as a natural food antioxidant. The antioxidant activity of honey however, varies greatly depending on the honey floral source. [12],[15] In several studies have shown that honeys have a rich phenolic profile consisting of benzoic acids and their esters, cinnamic acids and their esters and flavonoid aglycones. [16],[17],[18],[19] In general, the antioxidant capacity of honey appeared to be a result of the combined activity of a wide range of compounds including phenolics, peptides, organic acids, enzymes, and possibly other minor components. [11] Honey has been used in treatment different diseases as long ago as 2000 years. [20]
 Materials and Methods Materials and Methods | |  |
Peripheral blood samples obtained from seven FA patients (three boys and four girls) and seven healthy control children (two boys and five girls) were karyotyped. Both groups were aged between 7.5 and 19 years. The chromosomal culture method described by Rooney and Czepulkowski. [21] Three to five milliliter of sodium heparinized whole blood was collected from each patient and control individual. 0.5 cc of each patient and control individual's blood sample was added to 5 cc of a complete media containing RPMI 1640, fetal calf serum (10%), PHA (10 μg/ml), L- glutamate (2 mM), penicillin (200 unit/ml), and gentamycin (50 μg/ml). Each sample was cultured six times, the first was free, MMC solution was added to the second culture with a concentration of 40 ng/ml and the honey was added as a protective agent with different concentrations to the others cultures, two hours before adding MMC. After 70 hours of incubation in 37°C, colcemide was added (0.2 μg/ml). After 90 min, the cells were harvested by centrifugation (150 × g for 10 min). Then, 5 ml of 0.075 M KCl solution was added and mixed and incubated at 37°C for 15 min. After centrifugation (150 × g for 10 min), hypotonic supernatant was removed. Then, 5 cc cold, fresh fixative solution (3:1 methanol acetic acid) was added drop wise to the cell pellet. Centrifugation was done afterwards and the supernatant removed. These two latter steps were repeated until a clear pellet was obtained. Finally, cells obtained were dropped on distinct slides. Staining with Giemsa was performed for some of the slides prepared from each patient. Fifty metaphases, each from the MMC- treated and routinely cultured on both the patients and the normal controls, were analyzed for chromosomal breakage. The number of breaks per cell for each culture was calculated. The diagnosis of FA was done when only the MMC- induced breakages exhibited a significant increase in comparison to the controls. The effect of honey on MMC- induced chromosomal breakage was studied in lymphocytes from FA and healthy subjects.
 Results Results | |  |
Our results show that the mean spontaneous chromosomal breakage was 0.0666 ± 0.0208 in FA patients (n=7) and 0.0096 ± 0.00057 in the control group, (n=7). The difference between these two values was very significant (P = 0.009) [Table 1].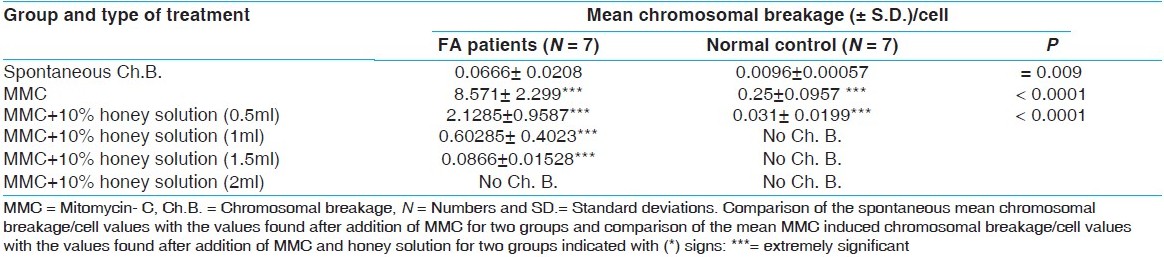 | Table 1: Comparison of the mean chromosomal breakage/cell of the Fanconi anemia patients and the normal group in lymphocyte cultures.
Click here to view |
The addition of MMC alone gave a significantly higher of chromosomal breakage in FA than control group (P = 0.0001) [Table 1] and [Figure 1]a and b. In the control group, when compared to spontaneous chromosomal breakages, when MMC was added to the cultures, mean chromosomal breakage/cell significantly increased to 0.25 ± 0.0957 from the spontaneous chromosomal breakage value of 0.0096 ± 0.00057 (P < 0.0001) [Table 1]. When the cell cultures were induced with MMC, the mean chromosomal breakage/cell frequencies of FA patients were found to be significantly higher (P < 0.0001) than the mean spontaneous chromosomal breakage value [Table 1].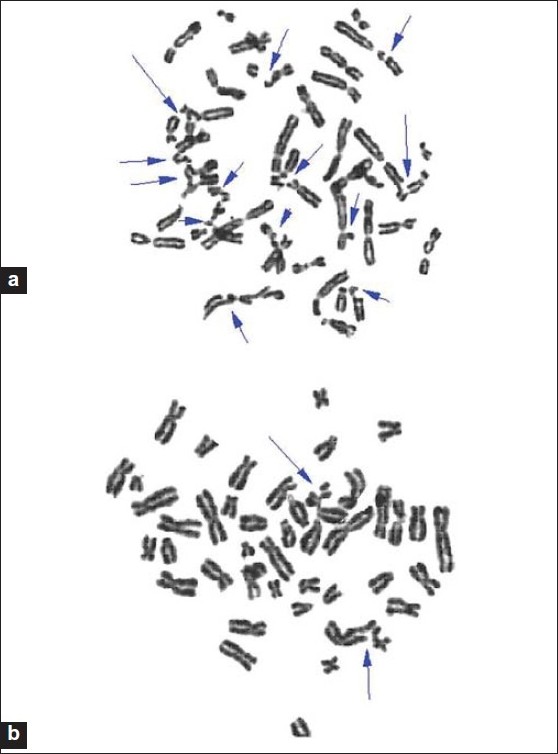 | Figure 1: (a) Metaphase spread from a Fanconi anemia patient showing multiple chromosomal breakages induced by mitomycin C (b) Metaphase spread from a healthy individual showing chromosomal breakages induced by mitomycin C.
Click here to view |
Addition of 10% honey aqueous solution and MMC together to the cultures decreased the mean chromosomal breakage/cell values in both groups when compared with the MMC induced chromosomal breakage frequencies [Table 1]. When the MMC and 0.5 ml of 10% honey solution added, cultures were compared in the two groups, the mean chromosomal breakage/cell was significantly higher (P < 0.0001) in the FA patients [Table 1]. But when the MMC and (1 and 1.5 ml) of 10% honey solution added, the mean chromosomal breakage/cell in FA group was (0.60285 ± 0.4023 and 0.0866 ± 0.01528, respectively), while, no chromosomal breakage in control group at these concentrations. Also, no chromosomal breakage in both groups when adding the MMC and 2 ml of 10% honey solution [Table 1]. When comparison of the mean MMC induced chromosomal breakage/cell values with the values found after addition of MMC and honey solution for two groups (indicated with (*) signs in [Table 1]) P< 0.0001, considered extremely significant. [Figure 2]a and b showed metaphases spread from Fanconi anemia patients after addition MMC and (0.5 and 2 ml of 10% honey aqueous solution, respectively).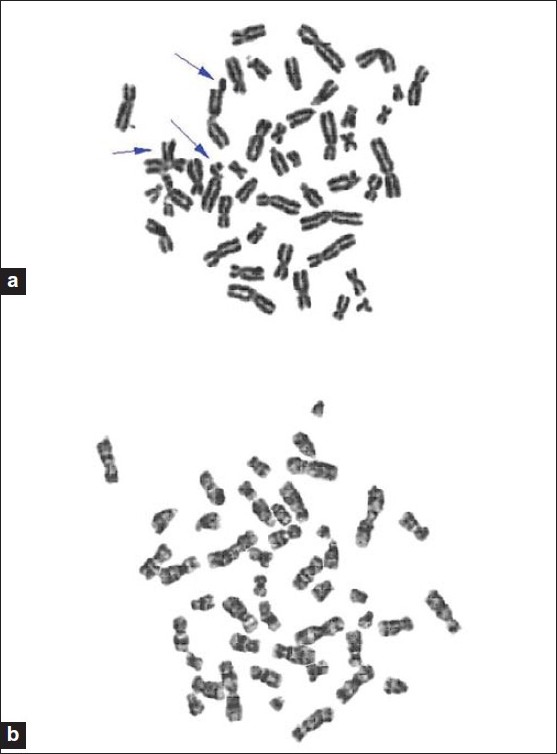 | Figure 2: (a) Metaphase spread from a Fanconi anemia patient showing decrease in chromosomal breakages by 0.5 ml of 10% honey aqueous solution against mitomycin C. (b) Metaphase spread from a Fanconi anemia patient showing protective effect of 2ml of 10% honey aqueous solution against mitomycin C induced chromosomal breakage.
Click here to view |
 Discussion Discussion | |  |
Oxidative stress, defined as an imbalance between the production of reactive oxygen species and antioxidant defense, is considered to be an important pathogenic factor in leukemia prone bone marrow diseases like FA. [22] Mohseni Meybodi and Mozdarani reported that net induced DNA damage by MMC was statistically higher and significantly different (P < 0.05) in FA patients than control group. [23] This observation agrees with our results where, the addition of MMC alone gave a significant higher of chromosomal breakage in FA than control group (P < 0.0001).
Our observation, seven FA patients (three boys and four girls), agrees with data from Tootian et al. suggesting that the ratio of females being affected was slightly higher than males. Comparison of several hematologic and clinical parameters in FA (MMC positive) and non FA (MMC negative) patients showed no clinically significant differences. [24] Many studies reported the genoprotective effect by enzyme supplementation or antioxidants. [25],[26],[27] However, a single report describes a reduction of chromosomal instability in a FA patient treated with a diet rich in antioxidants for these individuals. [28],[29]
Honey has been found to have a significant antioxidant content, [30] measured as the capacity of honey to scavenge free radicals, the antioxidant activity of honey has also been demonstrated. [31] The formation of the oxidant peroxide radical is catalyzed by metal ions such as iron and copper, and sequestering of these metal ions in complexes with organic molecules is an important antioxidant defense system. [32] Flavonoids (C 6 - C 3 - C 6 system) and other polyphenols, common constituents of honey, will do this. [33] The significant antioxidant effect of honey against damages induced by both water soluble and hydrophobic exogenous oxidants suggests that the ether fraction, owing to its lipophilic character, can interact with red blood cell membrane, and the protective effect can be associated with the binding of the flavonoids to the membrane. [34] These give an explanation for our observation, the basal chromosomal breakage count was higher among FA patients than healthy subjects and the addition of MMC alone gave a significantly higher of chromosomal breakage in FA patients than control group (P < 0.0001). While pre-treatment with honey significantly inhibited breakage induced by MMC in FA patients and control group by its antioxidant effect. Also, Akbulut et al.[35] observed that, significant correlations were obtained between the antioxidant activity of honey and phenolic contents. Mahgoub et al.[36] reported that, Honey dose dependently afforded protection against acetic acid- induced colonic damage. There was almost 100% protection with the highest dose of honey (5 g/kg) used while glucose, fructose, sucrose, maltose mixture produced no significant protective effect. As similar to this result, with the different method of using honey, our results showed that the highest dose of honey which gave 100% protection against MMC- induced chromosomal breakage in FA patients was (2 ml of 10% aqueous solution of honey) in vitro.
To our knowledge, this is the first study involving Honey as a cytoprotector for FA patients. We have shown that honey can prevent MMC- induced chromosomal breakage by its antioxidant effect.
 References References | |  |
| 1. | Joenje H, Patel KJ. The emerging genetic and molecular basis of Fanconi anemia. Nat Rev Genet 2001;2:446-57. 
|
| 2. | Auerbach AD, Buchwald M, Joenje H. The metabolic and molecular basis of inherited disease. In: Scriver C.R., et al, editors. New York: McGraw-Hill; 2001. p. 753-68. 
|
| 3. | Papadopoulo D, Moustacchi E. Fanconi anemia: Genes and function(s) revisited. Med Sci (Paris) 2005;21:730-6. 
|
| 4. | Levitus M, Joenje H, de Winter JP. The Fanconi anemia pathway of genomic maintenance. Cell Oncol 2006;28:3-29. 
|
| 5. | Auerbach AD, Rogatko A, Schroeder-Kurth TM. International Fanconi Anemia Registry: Relation of clinical symptoms to diepoxybutane sensitivity. Blood 1989;73:391-6. 
|
| 6. | Liu JM, Buchwald M, Walsh CE, Young NS. Fanconi anemia and novel strategies for therapy. Blood 1994;84:3995-4007. 
|
| 7. | Velazquez I, Alter BP. Androgens and liver tumors: Fanconi's anemia and non-Fanconi's conditions. Am J Hematol 2004;77:257-67. 
|
| 8. | Joenje H, Arwert F, Eriksson AW, de Koning H, Oostra AB. Oxygen-dependence of chromosomal aberrations in Fanconi's anaemia. Nature 1981;290:142-3. 
|
| 9. | Korkina LG, Deeva IB, De Biase A, Iaccarino M, Oral R, Warnau M, et al. Redox dependent toxicity of diepoxybutane and mitomycin C in sea urchin embryogenesis. Carcinogenesis 2000;21:213-20. 
|
| 10. | Raj AS, Heddle JA. The effect of superoxide dismutase, catalase and L-cysteine on spontaneous and on mitomycin C induced chromosomal breakage in Fanconi's anemia and normal fibroblasts as measured by the micronucleus method. Mutat Res 1980;78:59-66. 
|
| 11. | Gheldof N, Wang XH, Engeseth NJ. Identification and quantification of antioxidant components of honeys from various floral sources. J Agric Food Chem 2002;50:5870-7. 
|
| 12. | Gheldof N, Wang XH, Engeseth NJ. Buckwheat honey increases serum antioxidant capacity in humans. J Agric Food Chem 2003;51:1500-5. 
|
| 13. | Schramm DD, Karim M, Schrader HR, Holt RR, Cardetti M, Keen CL. Honey with high levels of antioxidants can provide protection to healthy human subjects. J Agric Food Chem 2003;51:1732-5. 
|
| 14. | Frankel S, Robinson GE, Berenbaum MR. Antioxidant content and correlated characteristics of 14 monofloral honeys. J Apic Res 1998;37:27-31. 
|
| 15. | Ferreres F, Ortiz A, Silva C, et al. Flavonoids of 'La Alcarria' honey. Z Lebensm Unters Forsch 1992;194:139-43. 
|
| 16. | Andrade P, Ferreres F, Amaral MT. Analysis of honey phenolic acids by HPLC, its application to honey botanical characterization. J Liq Chromatogr Relat Technol 1997;20:2281-8. 
|
| 17. | Ferreres F, Tomás-Barberán FA, Soler C, et al. A simple extractive technique for honey flavonoid HPLC analysis. Apidologie 1994;25: 21-30. 
|
| 18. | Martos I, Ferreres F, Tomás-Barberán FA. Identification of flavonoid markers for the botanical origin of Eucalyptus honey. J Agric Food Chem 2000;48:1498-502. 
|
| 19. | Tomás-Barberán FA, Martos I, Ferreres F, et al. HPLC flavonoid profiles as markers for the botanical origin of European unifloral honeys. J Sci Food Agric 2001;81:485-96. 
|
| 20. | Mathews KA, Binning AG. Wound management by using honey. Compend Con Edu 2002;24:23-59. 
|
| 21. | Rooney DE, Czepulkowski BH. Human Chromosome Preparation, Essential Techniques Series, D. Rickwood, Department of Biological and Chemical sciences, University of Essex, Wivenhoe Park, Colchester, UK. USA: University Press; 1997. p. 37-8. 
|
| 22. | Du W, Adam Z, Rani R, Zhang X, Pang Q. Oxidative stress in Fanconi anemia hematopoiesis and disease progression. Antioxid Redox Signal 2008;10:1909-21. 
|
| 23. | Meybodi A, Mozdarani H. DNA damage in leukocytes from Fanconi anemia patients and heterozygotes induced by mitomycin C and ionizing radiation as assessed by the comet and comet-FISH assay. Iran Biomed J 2009;13:1-8. 
|
| 24. | Tootian S, Mahjoubi F, Rahnama M, Hormozian F, Mortezapour F, Razazian F, et al. Cytogenetic investigation in Iranian patients suspected with Fanconi anemia. J Pediatr Hematol Oncol 2006;28:834-6. 
|
| 25. | Nordenson I. Effect of superoxide dismutase and catalase on spontaneously occurring chromosome breaks in patients with Fanconi's anemia. Hereditas 1977;86:147-50. 
|
| 26. | Raj AS, Heddle JA. The effect of superoxide dismutase, catalase and L-cysteine on spontaneous and on mitomycin C induced chromosomal breakage in Fanconi's anemia and normal fibroblasts as measured by the micronucleus method. Mutat Res 1980;78:59-66. 
|
| 27. | Ruppitsch W, Meisslitzer C, Hirsch-Kauffmann M, Schweiger M. Overexpression of thioredoxin in Fanconi anemia fibroblasts prevent the cytotoxic and DNA damaging effect of mitomycin C and diepoxybutane. FEBS Lett 1998;422:99-102. 
|
| 28. | Korkina LG, Samochatova EV, Maschan AA, Suslova TB, Cheremisina ZP, Afanas'ev IB. Release of active oxygen radicals by leukocytes of Fanconi anemia patients. J Leukoc Biol 1992;52:357-62. 
|
| 29. | Pagano G, Korkina LG. Prospects for nutritional interventions in the clinical management of Fanconi anemia. Cancer Cause Control 2000;11:881-9. 
|
| 30. | Frankel S, Robinson GE, Berenbaum MK. Antioxidant capacity and correlated characteristics of 14 unifloral honeys. J Apicultural Res 1998;37:27-31. 
|
| 31. | Mobarok Ali AT, al-Swayeh OA. Natural honey prevents ethanol-induced increased vascular permeability changes in the rat stomach. J Ethnopharmacol 1997;55:231-8. 
|
| 32. | HallIwell B, Cross CE. Oxygen-derived species: Their relation to human disease and environmental stress. Environ Health Perspect 1994;102 Suppl 10; 5-12. 
|
| 33. | Dailey LA, Imming P. 12-Lipoxygenase: Classification, possible therapeutic benefits from inhibition, and inhibitors. Curr Med Chem 1999;6:389-98. 
|
| 34. | Blasa M, Candiracci M, Accorsi A, Piacentini MP, Piatti E. Honey flavonoids as protection agents against oxidative damage to human red blood cells. Food Chem 2007;104:1635-40. 
|
| 35. | Akbulut M, Ozcan MM, Coklar H. Evaluation of antioxidant activity, phenolic, mineral contents and some physicochemical properties of several pine honeys collected from Western Anatolia. Int J Food Sci Nutr 2009;60:577-89. 
|
| 36. | Mahgoub AA, el-Medany AH, Hagar HH, Sabah DM. Protective effect of natural honey against acetic acid-induced colitis in rats. Trop Gastroenterol 2002;23:82-7. 
|
[Figure 1], [Figure 2]
[Table 1]
| This article has been cited by | | 1 |
Honey, chromosomal breakage and fanconi anemia |
|
| Wiwanitkit, S. and Wiwanitkit, V. | | Indian Journal of Human Genetics. 2012; 18(2): 269 | | [Pubmed] | |
|
 |
|






