|
 
 |
|
ORIGINAL ARTICLE |
|
|
|
| Year : 2011 | Volume
: 17
| Issue : 3 | Page : 218-225 |
| |
Growth response of Egyptian children with idiopathic short stature during four years of growth hormone therapy
Nagwa Abdallah Ismail1, Nermeen Salah Eldin Metwaly2, Fatma Ahmed El-Moguy3, Mona Hassan Hafez2, Soha M Abd El Dayem1, Tarek Mohamed Farid1
1 Department of Pediatrics, National Research Centre, Albhoss Str. Dokki, Giza, Egypt
2 Department of Pediatrics, Faculty of Medicine, Cairo University, Cairo, Egypt
3 Department of Clinical Pathology, Faculty of Medicine, Cairo University, Cairo, Egypt
| Date of Web Publication | 20-Jan-2012 |
Correspondence Address:
Nagwa Abdallah Ismail
National Research Centre, Albhoss Str. Dokki, Giza
Egypt
 Source of Support: None, Conflict of Interest: None
DOI: 10.4103/0971-6866.92102

 Abstract Abstract | | |
Background: Multiple factors affect the growth response to recombinant human growth hormone (rhGH) in children with idiopathic short stature (ISS).
Aim: To evaluate the growth responses of children with ISS treated with rhGH, aiming to identify the predictors of growth response.
Materials and Methods: We studied 120 cases, 90 males (75%), with a mean age of 13.8±2.7 years and 30 females (25%), with a mean age of 12.3±2.5 years. All patients received rhGH with a standard dose of 20 IU /m2 /week. The calculated dose per week was divided into six days and given subcutaneous at night.
Results: A significant positive trend was detected in the delta changes of all anthropometric data. For the first year, the growth response was positively correlated to CA and BA delay and negatively correlated to height, weight and IGF-1 SDSs. For the second year, the growth response was correlated positively to first year growth velocity, BA, triceps skin fold thickness SDS and deviation from target height, and negatively correlated to weight, IGFBP3 SDS and target height SDS. For the third year, the growth response was positively correlated to five variables namely target height, 2nd year growth velocity, IGF-1 SDS, weight SDS and triceps skin fold thickness SDS. For the fourth year, growth response was positively correlated to 2nd and 3rd year growth velocity, BA, deviation from target height and weight/ height SDS.
Conclusion: Our study showed multiplicity of predictors that is responsible for response in ISS children treated with rhGH, and BA was an important predictor.
Keywords: Adult height, bone age, GH therapy, idiopathic, puberty, short stature
How to cite this article:
Ismail NA, Metwaly NS, El-Moguy FA, Hafez MH, Abd El Dayem SM, Farid TM. Growth response of Egyptian children with idiopathic short stature during four years of growth hormone therapy. Indian J Hum Genet 2011;17:218-25 |
How to cite this URL:
Ismail NA, Metwaly NS, El-Moguy FA, Hafez MH, Abd El Dayem SM, Farid TM. Growth response of Egyptian children with idiopathic short stature during four years of growth hormone therapy. Indian J Hum Genet [serial online] 2011 [cited 2016 May 13];17:218-25. Available from: http://www.ijhg.com/text.asp?2011/17/3/218/92102 |
 Introduction Introduction | |  |
A serious effort has been made to come to an international consensus about the definition, sub classification of ISS, diagnosis and treatment. [1] The definition of ISS is based on the exclusion of other likely causes of short stature, as well as on the following minimal criteria: height less than third percentile or more than 2 SD below mean for age and sex, growth velocity below 10 th centile or less, bone age two or more years behind chronological age, normal findings from provocation GH tests (peak > 10 ng/ml) with no evidence of chronic organic disease nor psychological or severe emotional disturbance. [2] It is estimated that approximately 60-80% of all short children at or below -2 SDS fit the definition of ISS. [3] Multiple factors affect the growth response to GH, many of which are unknown. [1] Our aim is to evaluate the growth responses of children with ISS treated with rhGH, aiming to identify the predictors of growth response.
 Materials and Methods Materials and Methods | |  |
Patients
All patients were referred from different schools all over Egypt to the GH National Committee of the school health insurance, where they were diagnosed, provided by growth hormone and followed in association with the growth unit of the Diabetes Endocrine Metabolism Pediatric Unit (DEMPU), Children Hospital, Cairo University.
All patients had the inclusion criteria of a stature more than 2 SDS below the mean, growth velocity below the tenth centile for age and sex and a normal GH peak value (more than10 ng/ml) in at least one of the provocation tests. Children with dysmorphic phenotypes, such as skeletal dysplasias or Turner syndrome, and those with birth weight or length that are small for gestational age should be excluded from the ISS diagnostic category as are children with clearly identified causes of short stature (e.g. celiac disease, inflammatory bowel disease, juvenile chronic arthritis, GHD or GH resistance, hypothyroidism, Cushing's syndrome, etc.). They were 120 cases, 90 cases were males (75%), with a mean age of 13.8±2.7 years and 30 cases were females (25%), with a mean age of 12.3±2.5 years.
Methods
Informed consent was taken from the parents of children; then all cases were subjected to the following. Full history taking and clinical examinations were done. Full anthropometric assessment was also done, including target and mid-parental heights. Target height was calculated by the method of Tanner et al, taking the average of mother's and father's height after addition of 13 cm in boys or subtractions of them in girls, while mid-parental height is calculated as before ± 6.5 cm. [4]
Height was measured twice and neared to the next millimeter using Harpenden Stadiometer, height velocity in cm/year is the variable that describes the patient's one-year velocity and plotting it in the mid-year interval. Sitting height was also measured using Harpenden sitting height apparatus. Lower segment was calculated by subtraction of sitting height from height, and then from these two measurements, upper to lower segment ratio was derived (US/LS). Weight of the patients was measured using electronic balances and recorded in decimal of kilogram. Puberty was assessed by rating the breast development in girls, genital developments in boys, pubic and axillary hair development in both sexes, according to Tanner's classification. [5] All anthropometric procedures were performed at baseline before treatment and at follow-up by the same observer at the same time of the day (9 a.m.-1 p.m.) in the growth clinic of (DEMPU). Age-related normal standards for GHD patients were calculated from tables of Tanner and Whitehouse. [6] All the auxological data including estimated mature height (EMH) were analyzed by a software program (growth vision.2) provided by Novo-Nordisk, Denmark. Skeletal maturity was determined by the same observer from an X-ray of the left wrist and hand (Tanner Whitehouse no.2 method). Bone age delay, delta bone age and EMH were derived.
Laboratory investigations included the following:
- Thyroid profile (FT3, FT4, TSH) was done to exclude primary or secondary hypothyroidism as a cause of short stature. Thyroid stimulating hormone (TSH) was estimated by immunoradiometric assay (IRMA), while FT3 and FT4 were estimated by radioimmunoassay kits from Diagnosis Product Corporation, (Los angeles, CA, USA.)
- Routine general laboratory tests, if needed, which include complete blood picture, renal and liver function tests.
- GH secretion by two provocation tests (clonidine and insulin tolerance test) separated by one-week interval and analysis by immunoradiometric assay (IRMA). Dose of clonidine given before test was 0.15 mg/m 2 orally, while that of insulin was 0.1 U/kg I.V. Blood samples were drawn at 0, 20, 40, 60, 90, 120 and, sometimes at 180 min if hypoglycemia was delayed. Basal cortisol and at 60 min were, also, assessed after insulin stimulation.
Patients in pubertal age were primed with sex hormones prior to GH testing. Ethinyl estradiol was given in girls at a dose of 20 μg three times /day for three days, and in boys testosterone was given at a single dose of 100 mg three days preceding the test. - Insulin like growth factor-1 (IGF-1) and IGF binding protein-3 (IGFBP-3) were determined at diagnosis, by solid phase IRMA, using kits from Diagnostic System
Laboratories Inc. (Webster, TX, USA). DSL-5600 IGF-1 (IRMA) was included in a sample extraction step in which IGF-1 was separated from its binding protein in serum. This step is considered to be essential for accurate determination of IGF1. [7],[8]
Treatment protocol
All patients received biosynthetic growth hormone therapy. Three products are available in Egypt; Norditropin (Novo- Nordisk, Denmark), Genotropin (Pharmacia and Upjohn, Sweden) and Humatrope (Elli- Lilly, USA). All patients received rhGH with a standard dose of 20 IU /m 2 /week. The calculated dose per week was divided for six days and given subcutaneously at night. Puberty was not induced by giving sex hormones during GH treatment, since the treatment was started relatively late in these patients. All the patients accepted postponing induction of puberty after explanation by the physician.
Follow up
Patients were followed for a minimum period of one year and for a maximum of four years. Follow up for the 1 st year was achieved for 120 patients, in 2 nd year for 75 patients, in 3 rd year for 33 patients and lastly 21 patients were followed up in 4 th year.
The study group was followed every 3 months for anthropometric assessment, to assure compliance to therapy, to observe side effects and to renew the GH prescription. Follow up of thyroid profile, IGF-1and IGFBP-3 were done in every six months and skeletal maturity every year. All anthropometric procedures were performed at base line before treatment and at follow up by the same observer at the same time of the day (9am - 1pm) in the growth clinic of DEMPU.
Every year, the surface area of each patient was calculated, and the dose of GH was adjusted to keep the therapeutic dose at 20 IU /m 2 /week (equivalent to 0.2 mg/kg /week) for GHD. Response to GH therapy was judged on data obtained from auxological assessment, skeletal maturity, and estimated mature height.
Compliance to therapy is continuously verified by more than one parameter e.g. height velocity, asking the parents about mode of injection and dosing, counting the empty vials and sometimes by analysis of serum IGF-1.
The decision to stop GH treatment was when final adult height criteria was fulfilled and includes full pubertal development (Tanner stage 5), complete fusion of the epiphysis and growth velocity < 1 cm /year in the last year. Near final adult height is defined when the following criteria are achieved: Tanner stage 4 or more and bone age at least 14 years for females and 16 years for males.
The deviation of individual IGF-1 and IGFBP-3 values from the means for age and sex was calculated in standard deviation score (Z score) and subsequently used in statistical analysis. The laboratory of DEMPU, Cairo University children's Hospital, provided the mean values for IGF-1 and IGFBP-3.
Statistical analysis:
The SPSS software computer program was used for data analysis, and Harvard graphic for figures. Quantitative data were presented as mean ± SD, range, frequencies and qualitative data as percentage. For comparison of two groups, Student's t-test for dependent and independent variables was used. For comparison of more than two groups, analysis of variance (one way ANOVA) was used and followed by post hoc test when significant. P-value was considered as significant if it is less than or equal to 0.05.
Linear Pearson correlation was done followed by multiple linear regression, where the r value < 0.2 was considered as weak correlation, 0.2 to 0.5 was considered as mild correlation, 0.5 to 0.8 was considered as moderate correlation, and if r > 0.8, it was considered as strong correlation.
In order to identify predictors of growth response, linear regression analyses were performed (height velocity cm/year after 12 months of GH therapy was treated as dependant variable and demographic, auxological and biological parameters as independent variables). For each parameter, the result of the two-point yielding maximum r2 is included. Combination of parameters obtained at different time points was also examined in multiple regression models and then these predictors were ranked in order of importance and the percentage variability of the response explained by the predictors were demonstrated.
 Results Results | |  |
Descriptive statistic is presented in [Table 1] for patients with ISS.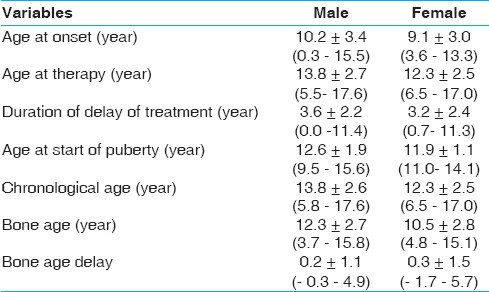 | Table 1: Descriptive statistics of patients with idiopathic short stature
Click here to view |
120 patients represent 19.2%, 90 (75%) males and 30 (25%) females with a mean age at onset of 9.9±3.3 years, CA at onset of therapy 13.4±2.7 years, BA at onset of therapy 11.8±2.8 years and BA delay of 1.6±1.2 years. The duration of delay of treatment (years) was 3.6±2.2 and 3.2±2.4 4 years in males and females of ISS respectively. [Table 2] shows anthropometric, skeletal maturity data of Patients with ISS. Height SDS was -3.7±1.0. Weight SDS was, -2.7±1. Weight for height SDS was 1.9±2.6. US/LS SDS was 0.1±1.4. Triceps SFT SDS was -0.5±0.9. Subscapular SFT SDS was, -0.2±0.9. Height SDS was improved from -3.7 to -2.6 in ISS after treatment. The patient's height became much closer to the target height as the difference changed from 32.1 to 25.7 cm (2.5 to 1.7 SDS) in ISS. Estimated mature height was improved from 156 to 163.6 cm in ISS, while the growth velocity was decreased from 7.6 cm (4 SDS) to 4 cm (0.4 SDS). 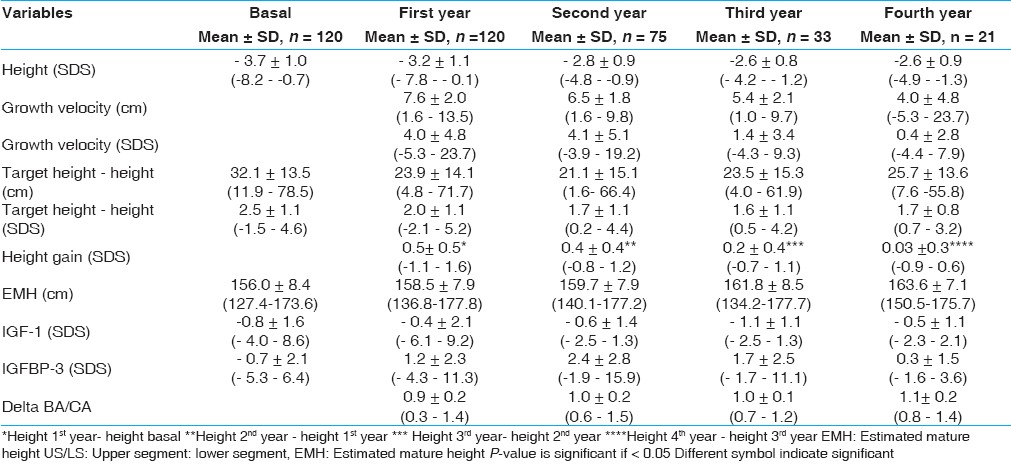 | Table 2: Anthropometric, skeletal maturity and laboratory data of patients with idiopathic short stature
Click here to view |
Prepubertal patients with ISS constituted 25% at onset of therapy decreased to 8.3%. The age of start of spontaneous puberty was 12.6±1.9 years and 11.9±1.1 years in males and females respectively.
For patients with ISS, there was a significant difference between prepubertal and patients undergoing puberty for 2 nd year delta BA/CA and 2 nd year height gain (P-value = 0.0001) for both. Also, there was a significant difference between pubertal patients and patients undergoing puberty for GV SDS of the 1 st and 2 nd years (P-value = 0.01 and 0.001) and for 2 nd year height gain (P-value = 0.0001), while there was a significant difference between prepubertal and pubertal patients for 2 nd year delta BA/CA (P-value = 0.0001) and 2 nd year GV SDS (P-value = 0.0001).
For the prediction of growth response in ISS during the four years of treatment, a regression equation is summarized in [Table 3] and [Table 4] where r2 was 0.37, 0.79, 1 and 1 with SD error (cm) of 4 and 2.4 for the 1 st , 2 nd , 3 rd and 4 th years, respectively.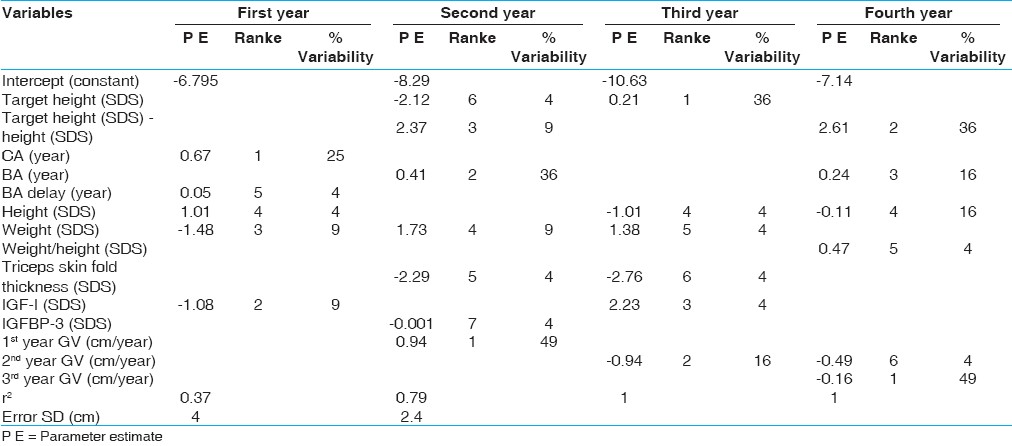 | Table 3: Regression equations for prediction of height velocity in all patients with idiopathic short stature
Click here to view |
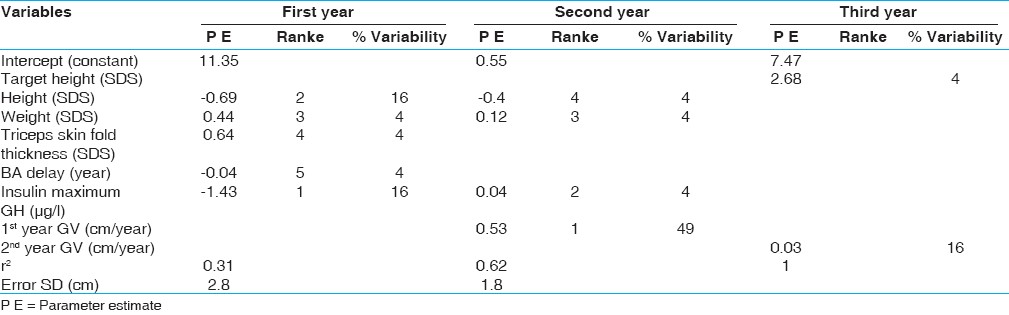 | Table 4: Regression equations for prediction of height velocity in prepubertal children with idiopathic growth hormone deficiency
Click here to view |
In patients with ISS, IGF-1 SDS increased from -0.8±1.6 at the onset of therapy to -0.4±2.1 after one year of GH treatment and IGFBP-3 SDS was increased from -0.7±2.1 to 1.2±2.3. For 21 patients with ISS followed for 4 years [Table 5], there was a significant difference between IGF-1 SDS in the 1 st year compared to 3 rd year (P- value = 0.008) and between IGFBP-3 SDS in the 1 st year and the 3 rd and 4 th years (P- value = 0.001).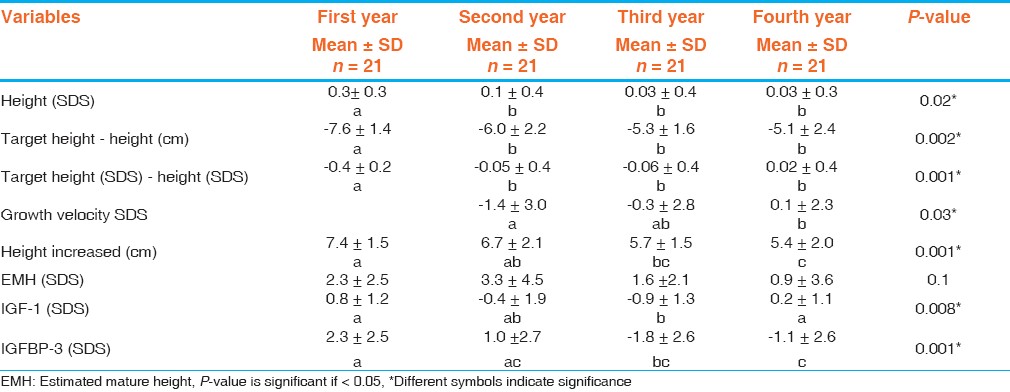 | Table 5: Delta change of auxological and laboratory data of patients with idiopathic short stature
Click here to view |
 Discussion Discussion | |  |
In children with ISS, short-term outcome measures (<2 years) must take into account the age, pubertal status, and degree of growth retardation of the individual patient. The change in height SDS will provide the best indicator of response, but height velocity, height velocity SDS, and the change in height velocity(cm per year or SDS) all have utility, and are sometimes superior, in assessing response. [1]
Long-term auxological parameters that define the success of therapy include adult height SDS, adult height SDS minus height SDS at start of GH, adult height minus predicted height, and adult height minus target height. [1]
In the present study, we included a cohort of 120 patients with idiopathic short stature. Our GH dose is in accordance with that used by Ranke (1996) in cases of ISS. [9] On GH therapy, patients showed catch up growth during 1 st and 2 nd years followed by a plateau response. The mean height SDS gains during 1 st , 2 nd , 3 rd and 4 th years during GH therapy were 0.5±0.5, 0.4±0.4, 0.2±0.4 and 0.03±0.3, also the height SDS gain during the first two years of GH therapy was 0.9±0.8 SDS.
Short-term auxological features that suggest a successful first year response to GH treatment in individual patients include a change in height SDS of more than 0.3- 0.5, a first-year height velocity increment of more than 3 cm/year, or a height velocity SDS of more than +1. Restoration to a more normal height during childhood is an important consideration. [10]
For 92 subjects who completed GH therapy for three years in Hopwood's study [11] the GV increased from 4.6 cm/year before treatment to a mean of 8.0, 7.6 and 7.2 during the first three years of GH therapy, which were higher compared to ours. The higher GV reported may be explained by the higher GH dose used (27 U/m 2 /w) compared to ours (20 U/m 2 /w).
It is of interest to mention that in our study a significant positive trend is observed in the delta changes of all anthropometric data of patients with ISS, including height and its SDS and difference between target height and patient's height except for the EMH where delta changes showed non- significant variation. This finding sets the discussion for the issue of the possible benefit of GH therapy in ISS patients regarding their final adult height. There has been much disagreement concerning the prognosis of adult height in children with ISS. This may be related to the lack of precise classification where some children seem to have a combination of genetic short stature and constitutional delay, while others seem to have a growth disorder based on their pathologic growth rate and height. Until there is a wide availability of genetic tests for mutation in the genes for GH and GH receptors, treatment decision will depend on a clinical judgment. [12]
In our patients with ISS, the mean age for spontaneous pubertal maturation was 11.9±1.1 years for girls and 12.6±1.9 years for boys. This to be compared with the mean age reported by Reckers et al, (11.4±0.9 years for girls, 13.1±1.4 years for boys) and Ranke and Lindberg, (12 years for girls and 12.6 years for boys). [13],[14]
In the present work, we tried to identify factors that may predict the growth response to GH treatment in this group of short children by applying a multiple linear regression analysis.
For the first year, the growth response was positively correlated to CA and BA delay and negatively correlated to height, weight and IGF-1 SDSs. These factors explained 37% of the variability with an error SD of 4 cm. For the second year, the growth response was correlated positively to first year growth velocity, BA, triceps skin fold thickness SDS and deviation from target height, and negatively correlated to weight, IGFBP3 SDS and target height SDS. These factors explained 79% of the variability with an error SD of 2.4 cm.
Other authors reported that children who are younger or heavier, who receive higher GH doses, and who are shortest relative to target height have the best growth response. These factors account for approximately 40% of the variance in growth response. Adult height outcome is influenced negatively by age at start and positively by midparental height, height at start, bone age delay, and the first-year response to GH. [15],[16]
Baseline and treatment-related IGF-I has not been validated in long-term studies, but two-year studies suggest that the rise in IGF-I correlates with short-term height gain. [17] IGF-I levels may be helpful in assessing compliance and GH sensitivity; levels that are consistently elevated (>2.5 SDS) should prompt consideration of GH dose reduction. [1]
For the third year, the growth response was positively correlated to five variables namely target height, 2 nd year growth velocity, IGF-1 SDS, weight SDS and triceps skin fold thickness SDS and was negatively correlated to height SDS at onset. For the fourth year, growth response was positively correlated to 2 nd and 3 rd year growth velocity, BA, deviation from target height and weight/ height SDS and negatively correlated to height SDS at onset. In the 3 rd and 4 th years of GH therapy these variables explained 100% all response variability.
In a longitudinal study of ISS subjects, bone age delay had an impact on the accuracy of prediction. In children with a bone age delay around two year, the average adult height was close to the predicted height, and in those with no bone age delay, adult height surpassed the initial prediction substantially, although if the bone age was delayed by more than two year, adult height was considerably below predicted height. [16]
In our patients with idiopathic short stature, only one female patient reached final adult height (147.9 cm, -2.2 SDS), whereas six patients (three males, three females) reached near final height of a mean of 157.8±4.7 cm (-2.8±0.6 SDS) for males and a mean of 144.7±10.2 cm (-2.4±1.6 SDS) for females.
The mean increase in adult height attributable to GH therapy (average duration of 4-7 year) in children with ISS is 3.5-7.5 cm compared with historical controls, [18],[19] with patients' own pretreatment predicted adult heights, [20] or with no treatment control or placebo control groups. [17],[21] Responses are highly variable and are dose dependent. Concern has been raised that higher GH doses (>53 μg/kg/d) may advance the bone age and the onset of puberty. [22]
The reason for the marked discrepancy between these data and the majority of the previously mentioned reports is the possibility of initiation of GH treatment at younger age.
It is apparent from our previous data that the multiplicity of predictors that is responsible for response variability in patients with ISS treated with GH, are lacking a characteristic pattern either in the period of catch up growth or in the period of stable growth. This confirms the complexity of growth response in ISS patients, with multidimensional parameters from different categories.
 References References | |  |
| 1. | Cohen P, Rogol AD, Deal CL, Saenger P, Reiter EO, Ross JL, et al. Consensus Statement on the Diagnosis and Treatment of Children with Idiopathic Short Stature: A Summary of the Growth Hormone Research Society, the Lawson Wilkins Pediatric Endocrine Society, and the European Society for Paediatric Endocrinology Workshop. J Clin Endocrinol Metab 2008;93:4210-7. 
|
| 2. | Ranke MB. Towards a consensus on the definition of idiopathic shortstature. Horm Res 1996;45(Suppl 2):64-6. 
|
| 3. | Lindsay R, Feldkamp M, Harris D, Robertson J, Utah RM. Growth Study: Growth standards and the prevalence of growth hormone deficiency. J Pediatr 1994;125:29-35. 
|
| 4. | Tanner JM, Goldstein H, Whitehouse RH. Standard for children's height at ages 2 to 9 years allowing for height of parents. Arch Dis Child 1970;45:755. 
|
| 5. | Tanner JM. The development of the reproductive system. In growth at adolescence. 2 nd ed. Oxford: Blackwell Scientific Publications; 1962. p. 50-5. 
|
| 6. | Tanner JM, Whitehouse RH, Marshal WA, Healy MJ Goldstein H. Assessment of skeletal maturity and prediction of adult height (TW2 method). London: Academic Press; 1985. 
|
| 7. | Le´ger J, Mercat I, Alberti C, Chevenne D, Armoogum P, Tichet J, et al. The relationship between the GH/IGF1 axis and serum markers of bone turnover metabolism in healthy children. Horm Res 2006;65:177. 
|
| 8. | Ranke MB, Blum WF, Bierich JR. Clinical relevance of serum measurements of IGFs and IGF binding proteins. Acta Paediatr Scand Suppl 1988;347:114-26. 
|
| 9. | Ranke MB. Towards a consensus on the definition of idiopathic short stature (Summary), Horm Res 1996;45(Suppl 2):64-6. 
|
| 10. | Ranke MB, Lindberg A, Price DA, Darendeliler F, Albertsson-Wikland K, Wilton P, et al. Age at growth hormone therapy start and first-year responsiveness to growth hormone are major determinants of height outcome in idiopathic short stature. Horm Res 2007;68:53-62. 
|
| 11. | Hopwood NJ, Hintz RL, Oertner JM. Growth response of children with non-growth hormone deficiency and marked short stature during three years of growth hormone therapy. J Pediatr 1993;123:215-22. 
|
| 12. | Takahashi Y, Kaji H, Okimura Y, Goji K, Abe H, Chichara K. Brief report: Short stature caused by a mutant growth hormone. N Engl J Med 1996;334:432-65. 
|
| 13. | Rekers-Mombarg LT, Massa GG, Wit JM, Matranga AM, Buckler JM, Butenandt O, et al. Growth hormone therapy with three dosage regimens in children with idiopathic short stature. J Pediatr 1998;132:455-60. 
|
| 14. | Ranke MB, Lindberg A, editors. Demographic characteristics of patients within KIGS: Special emphasis on sex and onset of puberty, international growth database, Biannual report No. 18, 2001. p. 1-9. 
|
| 15. | Cohen P, Rogol AD, Deal CL, Saenger P, Reiter EO, Ross JL, et al. Consensus statement on the diagnosis and treatment of children with idiopathic short stature: A summary of the Growth Hormone Research Society, the Lawson Wilkins Pediatric Endocrine Society, and the European Society for Paediatric Endocrinology Workshop. J Clin Endocrinol Metab 2008;93:4210-7. 
|
| 16. | Wit JM, Rekers-Mombarg LT; Dutch Growth Hormone Advisory Group. Final height gain by GH therapy in children with idiopathic short stature is dose dependent. J Clin Endocrinol Metab 2002;87:604-11. 
|
| 17. | Leschek EW, Rose SR, Yanovski JA, Troendle JF, Quigley CA, Chipman JJ, et al. Effect of growth hormone treatment on adult height in peripubertal children with idiopathic short stature: A randomized, double-blind, placebo-controlled trial. J Clin Endocrinol Metab 2004;89:3140-8. 
|
| 18. | Bryant J, Baxter L, Cave CB, Milne R. Recombinant growth hormone for idiopathic short stature in children and adolescents. Cochrane Database Syst Rev 2007;18:CD004440. 
|
| 19. | Hintz RL, Attie KM, Baptista J, Roche A; Genentech Collaborative Group. Effect of growth hormone treatment on adult height of children with idiopathic short stature. N Engl J Med 1999;340:502-7. 
|
| 20. | Wit JM, Rekers-Mombarg LT, Cutler GB, Crowe B, Beck TJ, Roberts K, et al. Growth hormone (GH) treatment to final height in children with idiopathic short stature: Evidence for a dose effect. J Pediatr 2005;146:45-53. 
|
| 21. | Finkelstein BS, Imperiale TF, Speroff T, Marrero U, Radcliffe DJ, Cuttler L. Effect of growth hormone therapy on height in children with idiopathic short stature: A meta-analysis. Arch Pediatr Adolesc Med 2002;156:230-40. 
|
| 22. | Kamp GA, Waelkens JJ, de Muinck Keizer-Schrama SM, Delemarre-Van de Waal HA, Verhoeven-Wind L, Zwinderman AH, Wit JM. High dose growth hormone treatment induces acceleration of skeletal maturation and an earlier onset of puberty in children with idiopathic short stature. Arch Dis Child 2002;87:215-20. 
|
[Table 5]
[Table 1], [Table 2], [Table 3], [Table 4]
|






