|
 
 |
|
ORIGINAL ARTICLE |
|
|
|
| Year : 2012 | Volume
: 18
| Issue : 3 | Page : 305-309 |
| |
The use of hormones indicators in human saliva in diagnosing parodontitis in pregnant women
SI Dolomatov1, W Zukow2, ID Atmazhov3, R Muszkieta4, A Skaliy2
1 Department of Biology, Odessa State Environmental University, Odessa, Ukraine
2 Department of Health and Physical Culture, University of Economy, Bydgoszcz, Poland
3 Medical Department, Odessa State Medical University, Odessa, Ukraine
4 Department of Health, Physical Culture and Tourism, Kazimerz Wielki University, Bydgoszcz, Poland
| Date of Web Publication | 4-Mar-2013 |
Correspondence Address:
W Zukow
University of Economy, Str. Garbary 2, 85-229 Bydgoszcz
Poland
 Source of Support: None, Conflict of Interest: None
DOI: 10.4103/0971-6866.107982

 Abstract Abstract | | |
Aims: The purpose of this work- was to study the dynamics of biochemical parameters of human saliva and analyze the features of the chemical composition of the saliva of women with abnormal pregnancy and in periodontitis against pregnancy.
Materials and Methods: The study included four groups of women: a control group of nonpregnant women of childbearing age (10), pregnant women with physiological pregnancy (24-28 weeks) without any signs of periodontal disease (10), pregnant with a generalized periodontitis I--II degrees in remission (10), women with pathological pregnancy with no signs of periodontal inflammation (10). In each of the groups over two samples of saliva were collected, the first collection of saliva in the morning on an empty stomach. Then mouthwash 0.9% sodium chloride solution was assigned and after 30 minutes the second portion of saliva. By enzyme immunoassay in samples of saliva of control groups of nonpregnant and pregnant women, as well as women with signs of a pathological course of pregnancy, the content of estriol, testosterone, and dehydroepiandrosterone sulfate was determined.
Statistical Analysis Used: Statistical data analysis was performed by the standard technique using Student's t-test.
Results: The results of biochemical analysis of saliva samples collected before rinsing the mouth with saline in groups of healthy nonpregnant and pregnant women were compared. It was established that during pregnancy the concentration of salivary estriol increases, but in pregnant women with periodontitis, the amount of this hormone in the saliva was significantly reduced. The highest content of testosterone in saliva samples, observed in healthy pregnant women, was significantly higher than nonpregnant women.
In pregnant women with periodontitis concentration of testosterone in saliva is reduced, while remaining significantly higher than its level in the saliva of nonpregnant women.
The highest concentration of testosterone is observed in the saliva of healthy pregnant women with periodontitis, but the smallest concentration of testosterone is found in the saliva of nonpregnant women. Also the nonpregnant group has the lowest levels of DHEA in pregnancy, and its content increases almost threefold when periodontal disease further grows.
Conclusions: It was established that periodontitis against pregnancy is characterized by higher levels of salivary DHEA sulfate and lower estriol, compared with a control group of pregnant women.
Keywords: Man, pregnancy, parodontitis, saliva
How to cite this article:
Dolomatov S I, Zukow W, Atmazhov I D, Muszkieta R, Skaliy A. The use of hormones indicators in human saliva in diagnosing parodontitis in pregnant women. Indian J Hum Genet 2012;18:305-9 |
How to cite this URL:
Dolomatov S I, Zukow W, Atmazhov I D, Muszkieta R, Skaliy A. The use of hormones indicators in human saliva in diagnosing parodontitis in pregnant women. Indian J Hum Genet [serial online] 2012 [cited 2016 Jun 1];18:305-9. Available from: http://www.ijhg.com/text.asp?2012/18/3/305/107982 |
 Introduction Introduction | |  |
Relevance of the study of the pathogenesis and course of periodontitis, [1] together with the development of new methods of its early diagnosis [2] is due, firstly, to the prevalence of this disease. [3] Second, the data that chronic periodontitis increases the risk to the health and lives in groups of patients with certain systemic diseases [4],[5] violate the physiological course of pregnancy. [6] Introduction of methods for early detection of disease based on biochemical analysis of saliva contributes to the minimization of risks. [2] Prospects for development of laboratory analysis of saliva is due to the improvement of noninvasive monitoring in practical medicine, including endocrinology [7] and pharmacology [8] and toxicology. [9] In normal human levels of some organic [10] and mineral [11] the components of saliva have fairly constant values, which greatly increase their diagnostic value. [12]
Thus, revealing of patterns of qualitative and quantitative changes in the biochemical composition of human saliva in health and disease is of great practical importance. [13] According to the results of clinical observations, noninvasive monitoring of pregnancy, based on laboratory analysis of saliva, deserves wider application in obstetric practice. [14]
The purpose of this work was to study the dynamics of biochemical parameters of human saliva and analysis features of the chemical composition of the saliva of women with abnormal pregnancy and in periodontitis against pregnancy.
 Materials and Methods Materials and Methods | |  |
This paper presents the results of four groups of women, including healthy pregnant women of reproductive age (20-35 years, 10), as well as pregnant women with physiological pregnancy (24-28 weeks) without any signs of periodontal disease (10) and with a generalized periodontitis I-II degrees in remission (10). In addition, the surveyed women with abnormal pregnancy without any signs of periodontal inflammation (10) were also included.
Violations of the physiological course of pregnancy were detected by measuring blood pressure, levels of human chorionic gonadotropin in serum, and ultrasound data. In each of the examined groups of patients and healthy subjects saliva samples were collected. Within 24 hours prior to the collection of saliva, in accordance with the published literature with guidance, [15] patients were recommended a diet that reduces nitrite load of the body and prevents entry of excess salt and animal proteins into the body.
Each subject collected two saliva samples: The collection of the first portion of saliva was performed on an empty stomach at 9.00 at rest in a sitting position. Then the subjects rinsed the mouth three times with 0.9% sodium chloride solution and 30 minutes after the mouthwash the second portion of saliva was collected. The saline solution was prepared in distilled water using chemically pure sodium chloride; the quantity of saline osmolality was 270 mOsm/kg H 2 O. In saliva samples collected after centrifugation for 15 at 3000 rpm using the enzyme immunoassay with standard test systems for in vitro diagnostic tests in saliva the content of the following hormones was determined: Estriol and testosterone (diagnostic kits, produced by Human, Germany), dehydroepiandrosterone sulfate (diagnostic kit manufactured by DRG, USA). Statistical data analysis was performed by the standard technique using Student's t-test.
 Results Results | |  |
The results of biochemical analysis of saliva samples collected before rinsing the mouth with saline in groups of healthy nonpregnant and pregnant women were compared. The results of the analysis of hormones in saliva to rinse a 0.9% NaCl solution are shown in [Table 1]. It was established that during pregnancy the concentration of salivary estriol increases, but in pregnant women with periodontitis, the amount of this hormone in the saliva was significantly reduced. The highest content of testosterone in saliva samples, observed in healthy pregnant women, was significantly higher than nonpregnant women. In pregnant women with periodontitis concentration of testosterone in saliva is reduced, while remaining significantly higher than its level in the saliva of nonpregnant women. Dynamics of DHEA in subjects is as follows: Its smallest concentration is noted in nonpregnant women; during pregnancy, its concentration in saliva increases; and in pregnant women with periodontitis, content of DHEA in saliva reaches the highest value. Further studies of samples of saliva were carried out after rinsing the mouth of women of all these groups with a solution of sodium chloride. The fact that the group of pregnant women with periodontitis shows the highest values of the rate of formation of saliva attracts more attention. After rinsing the mouth with 0.9% saline [Table 2], the dynamics of hormone levels in saliva has been tested in all of these groups and it keeps with the trends presented above. The concentration of estriol in saliva of healthy pregnant women is several times higher than that in nonpregnant women, but in periodontitis hormone levels significantly decreased, while remaining higher than in nonpregnant women.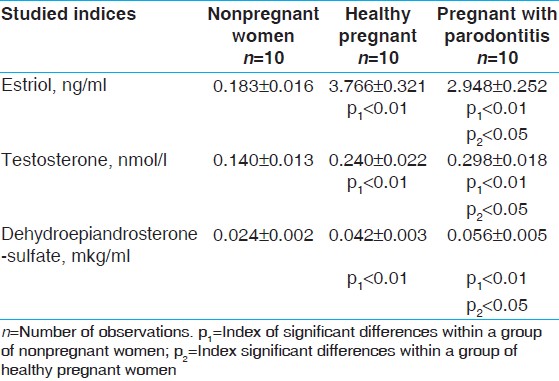 | Table 1: Contents of hormones in saliva of women with pathological course of pregnancy, pregnant with parodontitis, and healthy pregnant before rinsing of the mouth with salt solution (M±m)
Click here to view |
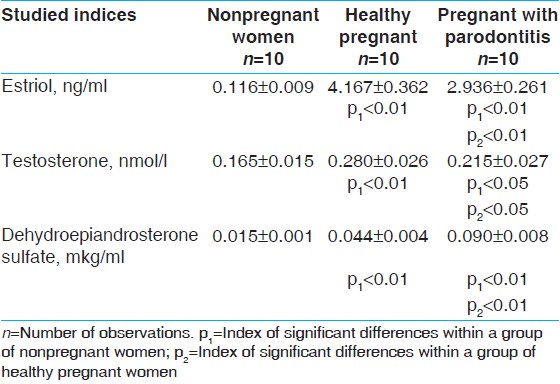 | Table 2: Contents of hormones in saliva samples collected after rinsing of the mouth with a salt solution in the group of women with pathological during pregnancy, pregnant with parodontitis, and healthy pregnant (M±m)
Click here to view |
The highest concentration of testosterone is observed in the saliva of healthy pregnant women with periodontitis, but the smallest concentration of testosterone is found in the saliva of nonpregnant women.
Also the nonpregnant group has the lowest levels of DHEA in pregnancy, and its content increases almost threefold when periodontal disease further grows.
 Discussion Discussion | |  |
In our opinion, as one of the most promising areas of research, the dynamics of steroid hormones in the saliva of pregnant women with periodontitis can be chosen to study while one is closer to solving the problems outlined. This conclusion is based on data from the literature that the physiological course of pregnancy is accompanied by increased production of estriol, dehydroepiandrosterone (DHEA), and other steroid hormones, synthesized by feto-placental complex and maternal adrenal glands. [16]
Amplification during pregnancy produces these hormones; most authors regard this as one of the main factors that create the conditions necessary for the adaptation of the female body. [17],[18],[19],[20] It was also reported that estrogens [21] and DHEA [22],[23] have distinct cytoprotective effects, reduce the damaging effects of inflammatory mediators in the tissue, and stimulate the protective reaction of the body. Not less important, in our opinion, are estrogens that have an important role in the regulation of metabolic and reparative processes of the epithelium lining the oral cavity of human. [24] In addition, clinical observations indicate that DHEA may be involved in protective and reparative processes of periodontium in periodontitis. [22]
Estriol - the main estrogen of pregnancy - was chosen as a marker of physiological pregnancy. DHEA - one of corticosteroids during pregnancy - plays a key role in the biosynthesis of estrogens feto-placental complex, [25] as evidenced by the high intensity of its absorption sincytotrophoblasts. [26] According to some authors, DHEA during pregnancy not only serves as an important substrate for the biosynthesis of estrogen, but also has its own regulatory effects required for successful gestation. [26],[27]
The analysis showed that samples of the saliva control group of pregnant women have a distinct increase in the concentration of estriol in comparison with nonpregnant against the backdrop of increasing concentrations of DHEA. Although periodontitis causes a moderate decrease in the concentration of estriol in saliva, compared with a control group of pregnant women, while the content of DHEA in the saliva of pregnant women with periodontitis increased.
According to the literature DHEA is the universal precursor of sex hormones, both male and female. [28] Therefore, it is permissible to consider increased synthesis and secretion of DHEA during pregnancy, amid falling clearance of sex hormones, including androgens, [29] as the main reasons for the increase of testosterone in the saliva of pregnant women.
The results of recent research show that DHEA is not only a substrate for the synthesis of sex hormones, but also has its own regulatory effects. [30],[31] It is proved that DHEA is involved in regulation of energy metabolism, [32] controls the activity of the hypothalamic-pituitary level that manages the endocrine status of the organism, [31] and develops atrial natriuretic peptide - a major humoral regulators volemic homeostasis. [33]
In addition, studies, in vitro, found that DHEA can modulate the output of the synthesis of nitric oxide endothelium of blood vessels [34] and angiogenesis. [35]
At present, it is found that DHEA synthesize the net area of the cortex of adrenal glands and, during pregnancy, feto-placental complex, influenced by sulfotransferaz converted into DHEA sulfate - the major transport form of the hormone, with its subsequent conversion back sulfatase in target tissues. [30],[36]
It is reported that the intensity of the absorption cell molecule DHEA sulfate is regulated by specific carrier proteins of organic anions and depends on the gradient of sodium and pH, as well as the level of activity of the sodium/potassium ATPase. [26] DHEA has the ability to inhibit the pathogenic mechanisms induced by mediators of inflammation. [28]
It is revealed that proinflammatory cytokines inhibit the activity of sulphotransferase and reduce their rate of biosynthesis. [30] Currently, the study of the pathway of steroid hormones in the tissues of the oral cavity is not enough, so we do not allocate ourselves the task of comparative analysis of levels of investigated hormones in blood plasma and saliva of pregnant women, because their metabolism in tissues, including organs of the oral cavity, can be characterized by a specific regional characteristics. Mechanisms that induce increased concentration of DHEA sulfate in samples of saliva of pregnant women with periodontitis, in our opinion, may be subject to more in-depth, independent research. In addition, attention is drawn to increase the hormone in the saliva of pregnant women with periodontitis under the influence of saline. Perhaps the increase in sodium chloride concentration in saliva during mouthwash brine affects the intensity of the sodium-dependent transport of the hormone in mouth. Indirect confirmation of this assumption is to reduce the concentration of osmotic active substantions in the saliva after rinsing the mouth with saline.
Given the antiinflammatory [28] and antioxidant [23] action of DHEA it may be assumed that the higher hormone levels in saliva samples collected in the group of pregnant women with periodontitis, before and after rinsing the mouth with saline, reflect a protective response in the body in response to the inflammation of periodontal tissues.
 Conclusions Conclusions | |  |
is established that periodontitis against pregnancy is characterized by higher levels of salivary DHEA sulfate and lower estriol, compared with a control group of pregnant women.
 References References | |  |
| 1. | Ryu OH, Choi SJ, Linares AM, Song IS, Kim YJ, Jang KT, et al. Gingival epithelial cell expression of macrophage inflammatory protein-1alpha induced by interleukin-1beta and lipopolysaccharide. J Periodontol 2007;78:1627-34. 
[PUBMED] |
| 2. | Christodoulides N, Floriano PN, Miller CS, Ebersole JL, Mohanty S, Dharshan P. Lab-on-a-chip methods for point-of-care measurements of salivary biomarkers of periodontitis. Ann N Y Acad Sci 2007;1098:411-28. 
|
| 3. | Hugoson A, Koch G, Göthberg C, Helkimo AN, Lundin SA, Norderyd O. Oral health of individuals aged 3-80 years in Jönköping, Sweden during 30 years (1973-2003). I. Review of findings on dental care habits and knowledge of oral health. Swed Dent J 2005;29:125-38. 
|
| 4. | Jacobs DR, Crow RS. Part V. Molecular and Protein Markers of Disease Subclinical Cardiovascular Disease Markers Applicable to Studies of Oral Health Multiethnic Study of Atherosclerosis. Ann NY Acad Sci 2007;1098:269-87. 
|
| 5. | Moutsopoulos NM, Madianos PN. Paradigm of Periodontal Infections. Ann NY Acad Sci 2006;1088:251-64. 
[PUBMED] |
| 6. | Offenbacher S, Boggess KA, Murtha AP, Jared HL, Lieff S, McKaig RG, et al. Progressive Periodontal Disease and Risk of Very Preterm Delivery. Obstet Gynecol 2006;107:29-36. 
[PUBMED] |
| 7. | Lopez ME, Colloca ME, Paez RG, Schallmach JN, Koss MA, Chervonagura A. Salivary characteristics of diabetic children. Braz Dent J 2003;14:26-31. 
|
| 8. | Torpet LA, Kragelund C, Reibel J, Nauntofte B. Oral adverse drug reactions to cardiovascular drugs. Crit Rev Oral Biol Med 2004;15:28-46. 
[PUBMED] |
| 9. | Koh D, Ng V, Chua LH, Yang Y, Ong HY, Chia SE. Can salivary lead be used for biological monitoring of lead exposed individuals?. Occup Environ Med 2003;60:696-8. 
|
| 10. | Ghafouri B, Tagesson C, Lindahl M. Mapping of proteins in human saliva using two-dimensional gel electrophoresis and peptide mass fingerprinting. Proteomics 2003;3:1003-15. 
[PUBMED] |
| 11. | McGehee JW, Johnson RB. Biomarkers of bone turnover can be assayed from human saliva. J Gerontol A Biol Sci Med Sci 2004;59:196-200. 
|
| 12. | Ozmeric N. Advances in periodontal disease markers. Clin Chim Acta 2004;343:1-16. 
[PUBMED] |
| 13. | Sculley DV, Langley-Evans SC. Periodontal disease is associated with lower antioxidant capacity in whole saliva and evidence of increased protein oxidation. Clin Sci 2003;105:167-72. 
[PUBMED] |
| 14. | Ramsey PS, Andrews WW. Biochemical predictors of preterm labor: Fetal fibronectin and salivary estriol. Clin Perinatol 2003;30:701-33. 
[PUBMED] |
| 15. | Mensinga TT, Speijers GJ, Meulenbelt J. Health implications of exposure to environmental nitrogenous compounds. Toxicol Rev 2003;22:41-51. 
[PUBMED] |
| 16. | Peter M, Dorr HG, Sippell WG. Changes in the concentrations of dehydroepiandrosterone sulfate and estriol in maternal plasma during pregnancy: A longitudinal study in healthy women throughout gestation and at term. Horm Res 1994;42:278-81. 
|
| 17. | Dean SA, Tan J, O'Brien ER, Leenen FH. 17{beta}-Estradiol downregulates tissue angiotensin-converting enzyme and ANG II type 1 receptor in female rats. Am J Physiol Regul Integr Comp Physiol 2005;288:R759-66. 
[PUBMED] |
| 18. | Harvey PJ, Morris BL, Miller JA, Floras JS. Estradiol Induces Discordant Angiotensin and Blood Pressure Responses to Orthostasis in Healthy Postmenopausal Women. Hypertension 2005;45:399-405. 
[PUBMED] |
| 19. | Owonikoko TK, Fabucci ME, Brown PR, Nisar N, Hilton J, Mathews WB, et al. In Vivo Investigation of Estrogen Regulation of Adrenal and Renal Angiotensin (AT1) Receptor Expression by PET. J Nucl Med 2004;45:94-100. 
[PUBMED] |
| 20. | Wu Z, Maric C, Roesch DM, Zheng W, Verbalis JG, Sandberg K. Estrogen Regulates Adrenal Angiotensin AT1 Receptors by Modulating AT1 Receptor Translation. Endocrinology 2003;144:3251-61. 
[PUBMED] |
| 21. | Simoncini T, Genazzani AR, De Caterina R. Towards a molecular understanding of the atheroprotective effects of estrogens: A review of estrogen effects on endothelial activation. Ital Heart J 2000;1:104-7. 
[PUBMED] |
| 22. | Ishisaka A, Ansai T, Soh I, Inenaga K, Yoshida A, Shigeyama C, et al. Association of Salivary Levels of Cortisol and Dehydroepiandrosterone With Periodontitis in Older Japanese Adults. J Periodontol 2007;78:1767-73. 
[PUBMED] |
| 23. | Ripp SL, Falkner KC, Pendleton ML, Tamasi V, Prough RA. Regulation of CYP2C11 by Dehydroepiandrosterone and Peroxisome Proliferators: Identification of the Negative Regulatory Region of the Gene. Mol Pharmacol 2003;64:113-22. 
[PUBMED] |
| 24. | Välimaa H, Savolainen S, Soukka T, Silvoniemi P, Mäkelä S, Kujari H, et al. Estrogen receptor-beta is the predominant estrogen receptor subtype in human oral epithelium and salivary glands. J Endocrinol 2004;180:55-62. 
|
| 25. | Barker EV, Hume R, Hallas A, Coughtrie WH. Dehydroepiandrosterone sulfotransferase in the developing human fetus: Quantitative biochemical and immunological characterization of the hepatic, renal, and adrenal enzymes. Endocrinology 1994;134:982-9. 
|
| 26. | Zhou F, Tanaka K, Soares MJ, You G. Characterization of an organic anion transport system in a placental cell line. Am J Physiol Endocrinol Metab 2003;285:E1103-9. 
|
| 27. | Kroboth PD, Salek FS, Pittenger AL, Fabian TJ, Frye RF. DHEA and DHEA-S: A review. J Clin Pharmacol 1999;39:327-48. 
|
| 28. | Iwasaki Y, Asai M, Yoshida M, Nigawara T, Kambayashi M, Nakashima N. Dehydroepiandrosterone-Sulfate Inhibits Nuclear Factor- B-Dependent Transcription in Hepatocytes, Possibly through Antioxidant Effect. J Clin Endocrinol Metab 2004;89:3449-54. 
|
| 29. | Kerlan V, Nahoul K, Le Martelot MT, Bercovici JP. Longitudinal study of maternal plasma bioavailable testosterone and androstanediol glucuronide levels during pregnancy. Clin Endocrinol (Oxf.) 1994;40:263-7. 
|
| 30. | Reed MJ, Purohit A, Woo LW, Newman SP, Potter BV. Steroid Sulfatase: Molecular Biology, Regulation, and Inhibition. Endocr Rev 2005;26:171-202. 
|
| 31. | Young J, Couzinet B, Nahoul K, Brailly S, Chanson P, Baulieu EE, et al. Panhypopituitarism as a Model to Study the Metabolism of Dehydroepiandrosterone (DHEA) in Humans. J Clin Endocrinol Metab 1997;82:2578-85. 
|
| 32. | Sandoval DA, Ping L, Neill RA, Morrey S, Davis SN. The effects of dehydroepiandrosterone sulfate on counterregulatory responses during repeated hypoglycemia in conscious normal rats. Diabetes 2004;53:679-86. 
|
| 33. | Moriyama Y, Yasue H, Yoshimura M, Mizuno Y, Nishiyama K, Tsunoda R, et al. The Plasma Levels of Dehydroepiandrosterone Sulfate Are Decreased in Patients with Chronic Heart Failure in Proportion to the Severity. J Clin Endocrinol Metab 2000;85:1834-40. 
|
| 34. | Simoncini T, Mannella P, Fornari L, Varone G, Caruso A, Genazzani AR. Dehydroepiandrosterone Modulates Endothelial Nitric Oxide Synthesis Via Direct Genomic and Nongenomic Mechanisms. Endocrinology 2003;144:3449-55. 
|
| 35. | Liu D, Dillon JS. Dehydroepiandrosterone Activates Endothelial Cell Nitric-oxide Synthase by a Specific Plasma Membrane Receptor Coupled to Gα1,2,3. J Biol Chem 2002;277:21379-88. 
|
| 36. | Strott Ch A. Sulfonation and Molecular Action. Endocr Rev 2002;23:703-32. 
|
[Table 1], [Table 2]
|






