|
 
 |
|
ORIGINAL ARTICLE |
|
|
|
| Year : 2012 | Volume
: 18
| Issue : 3 | Page : 340-343 |
| |
Analysis of loss of heterozygsity effect on thyroid tumor with oxyphilia cell locus in familial non medullary thyroid carcinoma in Iranian families
Hasti Atashi Shirazi1, Mehdi Hedayati1, Maryam Sadat Daneshpour1, Abdollah Shafiee2, Fereidoun Azizi3
1 Cellular Molecular and Endocrine Research Center, Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran
2 Milad hospital, Hakim highway, Tehran, Iran
3 Cellular Molecular and Endocrine Research Center; Endocrine Research Center, Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran
| Date of Web Publication | 4-Mar-2013 |
Correspondence Address:
Fereidoun Azizi
Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, PO Box 19195 - 4763, Tehran
Iran
 Source of Support: None, Conflict of Interest: None
DOI: 10.4103/0971-6866.107989

 Abstract Abstract | | |
Material and Methods: 22 nuclear families (78 persons including 12 patients) with papillary and follicular tumors were selected in a period of six months from Milad hospital. Five microsatellite markers (D19S413, D19S391, D19S916, D19S568, D19S865) on 19p13.2 were selected for genetic analysis. Genomic DNAs was extracted; PCR and polyacrylamide gel electrophoresis method were used for variation detection.
Results: The results show that 5.4% of the follicular carcinomas and 17.9% of the papillary carcinomas presented LOH at recognition sites. LOH of Papillary carcinoma detected about 13.9% and follicular carcinoma 7.2% in this study. The frequency of informative cases was not similar for each marker: D19S413 (41.1%)[1], D19S391 (12.5%), D19S916 (10.7%), D19S568 (1.8%) and D19S865 (3.6%). Loss of hetrozygosity in D19S413 predicts the relation between variation in this region and the disease.
Discussion: Our findings showed an average of 13.9% LOH in FNMTC cases. Among the five major microsatellites, D19S413 was the most informative for LOH analysis of FNMTC.
Keywords: Familial non medullary thyroid carcinoma, Iran, loss of heterozygosis, thyroid tumor with oxyphilia cell
How to cite this article:
Shirazi HA, Hedayati M, Daneshpour MS, Shafiee A, Azizi F. Analysis of loss of heterozygsity effect on thyroid tumor with oxyphilia cell locus in familial non medullary thyroid carcinoma in Iranian families. Indian J Hum Genet 2012;18:340-3 |
How to cite this URL:
Shirazi HA, Hedayati M, Daneshpour MS, Shafiee A, Azizi F. Analysis of loss of heterozygsity effect on thyroid tumor with oxyphilia cell locus in familial non medullary thyroid carcinoma in Iranian families. Indian J Hum Genet [serial online] 2012 [cited 2016 Jun 1];18:340-3. Available from: http://www.ijhg.com/text.asp?2012/18/3/340/107989 |
 Introduction Introduction | |  |
Thyroid cancers, like other human tumors, arise from a single transformed cell that presumably gained growth advantage through damage to genes that control cellular proliferation. [1] As the genome becomes unstable, the cell is more prone to developing large scale chromosome aberrations, amplifications, deletions, and translocations. The subsequent accumulation of genetic abnormalities is associated with increased genetic heterogeneity in the tumor clone and with more aggressive and invasive behavior. A frequently measured manifestation of chromosomal instability is allelic deletions, primarily detected using highly polymorphic markers for which samples lose one of the heterozygous bands. [2]
Known environmental risk factors for non-medullary thyroid carcinoma (NMTC) are dietary iodine deficiency and excessive exposure to radiation during childhood. [3] TCO, mapped to chromosome 19p13.2, by linkage analysis with a whole genome panel of microsatellite markers, is responsible for predisposition to thyroid tumors. [4],[5] There is substantial controversy about the classification and behavior of tumors with cell oxyphilia. [4],[5]
Although most NMTC are thought to be sporadic, several studies have reported a four to nine fold increased risk of thyroid cancer in relatives of patients and two fold increased risk in women. [6] The prevalence of familial cases among all NMTC cases ranges from 3.5-6.2 percent. [7],[8],[9] The clustering of NMTC in a family may be the result of a known predisposition to cancer such as familial adenomatous polyposis (FAP) or Cowden disease. Conversely, several families have been reported with clustering of NMTC without evidence of an identifiable familial cancer syndrome. [7] NMTC in these families is often bilateral or multifocal and occurs at an earlier age than the sporadic form. It is associated with benign thyroid pathology, and may have a more aggressive course. [6],[7],[8] This research has extended for deletion of the sporadic oxyphilic tumors through the study of LOH and led to the discovery of LOH in the 19q31.2 region (TCO).
 Materials and Methods Materials and Methods | |  |
This study included patients with pathologically confirmed NMTC diagnosed. A clinical questionnaire was also proposed to patients, and their pedigrees were constructed. By using the standard salting out protocol, DNA material extracted from 78 blood samples collected from patients and their families and one normal DNA as a control. The DNA samples were genotyped using microsatellite markers with their Marshfield regions (D19S391 28.8 cM, D19S916 31.3 cM, D19S413 32.4 cM, D19S865 32.4 cM, and D19S568 50.8 cM) for 19p13.2. Genetic distances were taken from Marshfield genetic maps and the genetic distance database. The primer sequences were obtained from public databases (markers of chromosomes 19) and the literatures of markers for chromosome 19 [2],[4] that shown in [Table 1]. PCR reactions were carried out in a volume of 25 μL (2.5x buffer PCR, 2 μL MgCl 2 , and 0.5 μL of deoxy-NTP, 1 μL DNA, 1 μL of each primer and 25 μL of mineral oil). The reactions were carried out in Hybaid PCR system with the following thermal profile: Denaturation at 95°C for 5 min; 32 cycles of 94°C for 30 mins, 53°C (or 55°C) for 1 min, and 72°C for 1 min, with a final extension at 72°C for 10 min. The products of PCR were detected by electrophoresis on 1.5% agarose and then on 8% acrylamide gels for 3-6 h at 45-50°C. Allelic fragments were visualized by autoradiography. Definition of the allelic loss was limited to the informative cases. In the LOH technique, a given case was considered informative for a given microsatellite marker when the corresponding genomic DNA was heterozygous, and thus produced two bands in the gel, namely the maternal and the paternal allele. Non-informative cases, therefore, were the inverse scenario. The signal intensity of the polymorphic alleles was evaluated visually by three reviewers blindly and in an independent fashion. Thus, a sample had LOH when one of the two bands visible in the simultaneously tested genomic DNA was absent or had its signal intensity reduced by more than 50 percent. In this study informative data means that the data allows the researcher to obtain valuable information with respect to basic model structure and is a requirement on the experiment design.
 Results Results | |  |
One candidate locus (TCO) for the 78 members was examined in present study. Individuals showing papillary thyroid carcinoma (PTC) and follicular thyroid carcinoma (FTC) were considered as affected for analysis. Selected regions were analyzed for 78 persons who included 12 patients affected by thyroid carcinomas. Three persons were affected by FTC and nine were affected by PTC. We observed 5.4% of the FTC, and 17.9% of the PTC presented LOH at recognition sites. The frequency of informative cases was not similar for each marker: D19S413 (41.1%), D19S391 (12.5%), D19S916 (10.7%), D19S568 (1.8%) and D19S865 (3.6%).
Among the 5 major microsatellites, D19S413 was the most informative for LOH analysis of NMTC; alterations included allelic loss in a gene block that is observed in patients as well as their families. Indetermination of loss in heterozygous samples was done by comparing the intensity of the alleles in DNA from the patients' blood to normal. If the ratio of the alleles in patients' blood DNA was half of the ratio of the normal DNA, it was considered an allelic loss. Gain constitutional homozygosity was regarded as non-informative. Among the informative cases, heterozygosity at all five loci was retained (i.e., LOH was absent) in all normal tissues [Figure 1] and [Figure 2]. The specific haplotype that was co-inherited with the disease were analyzed and that was not shared by the unaffected family members. It was compared between PTC, FTC and LOH with variables by five markers [Figure 1],[Figure 2],[Figure 3] and [Figure 4].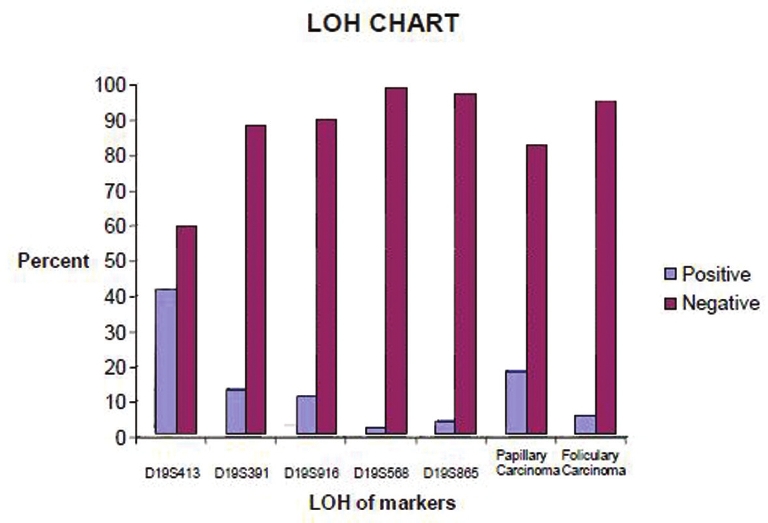 | Figure 1: LOH analysis of informative cases by five microsatellite markers in patients and their families and also papillary and follicular carcinoma in the study
Click here to view |
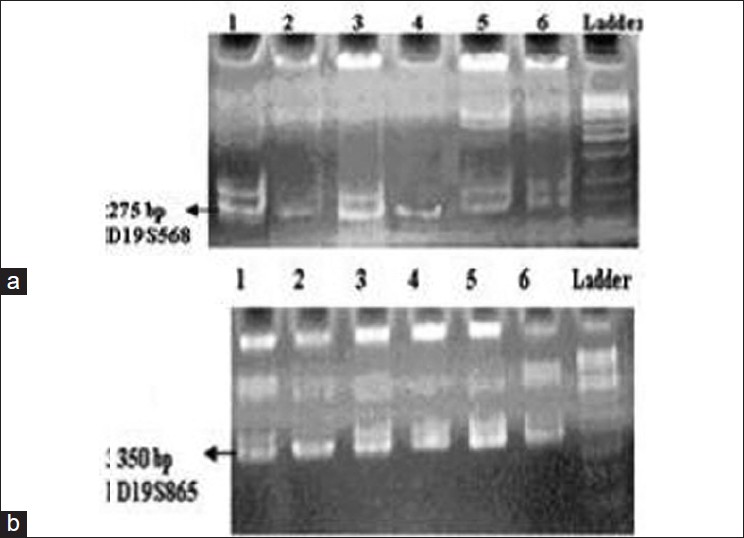 | Figure 2: Acrylamide gel, PCR result and detected LOH; a. chromosomes 19p13.2 sample 1: control, sample 2: patient, sample 3: her husband and samples 4, 5, and 6 are her children; b. chromosomes 19p13.2 sample 2: patient; 3: his wife, 1: control and 4, 5 and 6 are his children
Click here to view |
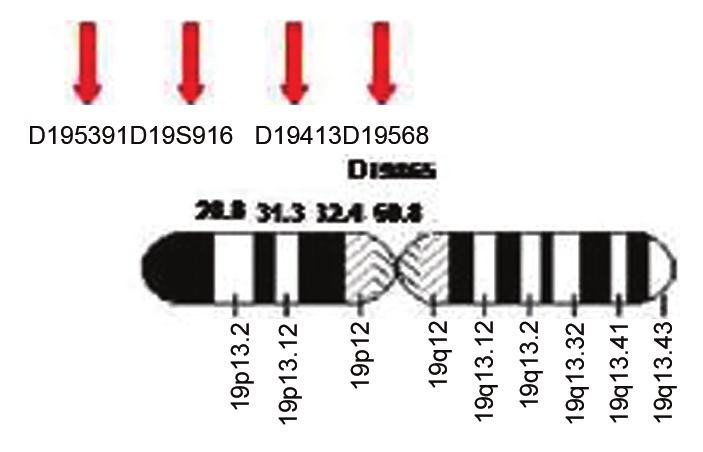 | Figure 3: Location of 5 markers on chromosome 19 with their Marshfield regions
Click here to view |
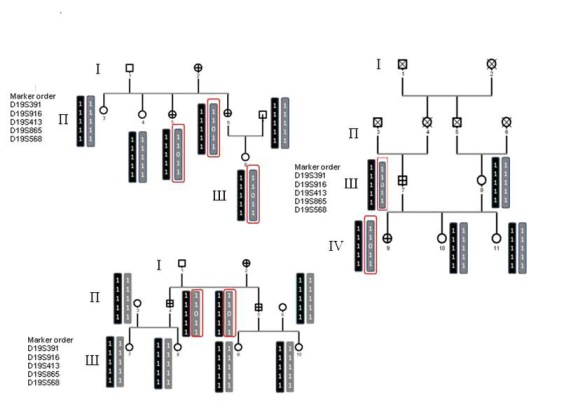 | Figure 4: Pedigree of family papillary and follicular, with haplotype analysis in the region of linkage to susceptibility to thyroid cancer and TCO, on chromosome 19. Circles represent females, and squares represent males; crossed symbols denote cases of thyroid cancer. Critical recombination in individuals II-2, II-3 and II-4 define a 1 - 1-0 - 1-1 haplotype that is co- inherited with the disease and that is not shared by the unaffected family members
Click here to view |
 Discussion Discussion | |  |
In the present study, we expanded the analysis of 19p13.2 locus and also included a significant number of follicular and papillar carcinomas. We found that LOH in D19S413 occurs more frequently in some patients and their families according of a gene block that in turn is inherited by the next generations.
The genetic background of FNMTC is still poorly understood but, fortunately loss of genetic material in thyroid neoplasm has been studied by cytogenetic and LOH analysis. Most reports on large kindred suggest an autosomal dominant inheritance with variable penetrance but other models of inheritance are also possible, including polygenic predisposition. [9],[10] From the haplotype analysis, it appears that these patients transmit the mutated TCO allele to their progenies which is not the allele deleted in sample, thus suggesting that TCO might be a tumor suppressor gene. Loss of function in tumor suppressor genes requires structural or functional inactivation of both alleles. One of these is frequently lost as part of a large deletion of chromosomal material. Therefore, efforts to identify consistent regions of chromosome loss in a given tumor type are a standard way to localize and eventually identify tumor suppressor genes. Higher rates of LOH in many cases of FTC have been reported previously on chromosomes 11, 16, 3, 2, 10, and 1. [11]
Our findings suggest that hereditary or other familial factors are important in a small proportion of NMTC. The tumors tend to be multifocal and may invade locally, then metastasize to regional lymph nodes while they are relatively small. Our study displays the importance of obtaining a family history for patients with NMTC, as hereditary factors are important in a small proportion percentage of these patients. It is possible that the nature of the tumor-initiating oncogenic event may be significant in determining the genomic stability of the tumor and this may have implications for tumor behavior and prognosis or somatic inactivation of the putative susceptibility gene, located at 19p13.2, has occurred through the other mechanisms, such as microdeletions, point mutation, or promoter hyper-methylation and interact with this region. The study of the genetics of FNMTC is an exciting field in medical research that has the potential to permit individualized management of thyroid cancer. Careful follow-up for patients with a family history of thyroid cancer through routine physical examinations is critical, as clinical differences between sporadic and familial cases may be present. Some genes like MNG1, TCO1, PTCPRN, PTEN, TSHR, TRKA and SLC5A5 (sodium-iodide symporter) play role in familial nonmedullary thyroid cancer and also 6 candidate genes (RET, MET, TSHR, APC, and PTEN) were also excluded using microsatellite markers positioned in or in close proximity to TCO locus. We know each gene has individual effect and might be deletion of these named genes results in happening LOH in TCO and it demands for further investigations on diagnosis and sufficient analytical power as well.
The study of TCO locus was not sufficient enough to provide statistically significant conclusions in all samples analyzed. Sufficient analytical of other genes that play role in familial non-medullary thyroid cancer should be done in next steps and the limitation of time did not permit.
Molecular studies are necessary to determine the inherited predisposition to cancer in these families as well as on the prevalence of neoplasms at other sites and survival of familial cases. Since these results have been contradictory, further large-scale genetic studies utilizing emerging molecular screening tests are necessary to elucidate the underlying genetic basis of FNMTC.
 References References | |  |
| 1. | Bast R., et al. Holland-Frei Cancer Medicine. 5 th ed. Hamilton (ON): BC Decker; 2000. 
|
| 2. | Canzian F, Amati P, Harach HR, Kraimps JL, Lesueur F, Barbier J, et al. A gene predisposing to familial thyroid tumors with cell oxyphilia maps to chromosome 19p13.2. Am J Hum Genet 1998;63:1743-8. 
|
| 3. | Ward LS, Brenta G, Medvedovic M, Fagin JA. Studies of allelic loss in thyroid tumors reveal major differences in chromosomal instability between papillary and follicular carcinomas. J Clin Endocrinol Metab 1998;83:525-30. 
|
| 4. | Lesueur F, Stark M, Tocco T, Ayadi H, Delisle MJ, Goldgar DE, et al. Genetic heterogeneity in familial nonmedullary thyroid carcinoma: Exclusion of linkage to RET, MNG1, and TCO in 56 families. NMTC Consortium. J Clin Endocrinol Metab 1999;84:2157-62. 
|
| 5. | Gasparre G, Bonora E, Tallini G, Romeo G. Molecular features of thyroid oncocytic tumors. Mol Cell Endocrinol 2010;321:67-76. 
|
| 6. | Malchoff CD, Malchoff DM. The genetics of hereditary nonmedullary thyroid carcinoma. J Clin Endocrinol Metab 2002;87:2455-9. 
[PUBMED] |
| 7. | Pal T, Vogl FD, Chappuis PO, Tsang R, Brierley J, Renard H, et al. Increased risk for nonmedullary thyroid cancer in the first degree relatives of prevalent cases of nonmedullary thyroid cancer: A hospital-based study. J Clin Endocrinol Metab 2001;86:5307-12. 
|
| 8. | Prazeres HJ, Rodrigues F, Soares P, Naidenov P, Figueiredo P, Campos B, et al. Loss of heterozygosity at 19p13.2 and 2q21 in tumours from familial clusters of non-medullary thyroid carcinoma. Fam Cancer 2008;7:141-9. 
|
| 9. | Malchoff CD, Malchoff DM. Familial nonmedullary thyroid carcinoma. Cancer Control 2006;13:106-10. 
|
| 10. | Bonora E, Tallini G, Romeo G. Genetic Predisposition to Familial Nonmedullary Thyroid Cancer: An Update of Molecular Findings and State-of-the-Art Studies. J Oncol 2010;2010:385206. 
|
| 11. | Grebe SK, McIver B, Hay ID, Wu PS, Maciel LM, Drabkin HA, et al. Frequent loss of heterozygosity on chromosomes 3p and 17p without VHL or p53 mutations suggests involvement of unidentified tumor suppressor genes in follicular thyroid carcinoma. J Clin Endocrinol Metab 1997;82:3684-91. 
|
[Figure 1], [Figure 2], [Figure 3], [Figure 4]
[Table 1]
|






