|
 
 |
|
ORIGINAL ARTICLE |
|
|
|
| Year : 2013 | Volume
: 19
| Issue : 2 | Page : 239-244 |
| |
Association between PRO12ALA polymorphism of the PPAR-γ2 gene and type 2 diabetes mellitus in Iranian patients
Azadeh Motavallian1, Sasan Andalib2, Golnaz Vaseghi3, Hamid Mirmohammad-Sadeghi4, Masoud Amini5
1 Department of Pharmacology, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran
2 Neurosciences Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
3 Physiology Research Center, Isfahan University of Medical Sciences, Isfahan, Iran
4 Department of Biotechnology, School of Pharmacy and Pharmaceutical Sciences, Isfahan University of Medical Sciences, Isfahan, Iran
5 Endocrine and Metabolism Research Center, Sedigheh Tahereh Medical Research Complex, Isfahan, Iran
| Date of Web Publication | 5-Aug-2013 |
Correspondence Address:
Hamid Mirmohammad-Sadeghi
Department of Biotechnology, School of Pharmacy and Pharmaceutical Sciences, Isfahan University of Medical Sciences, Isfahan
Iran
 Source of Support: None, Conflict of Interest: None  | 6 |
DOI: 10.4103/0971-6866.116126

 Abstract Abstract | | |
Background: Peroxisome proliferator-activated receptor (PPARs) have been identified as ligand-activated transcription factors that belong to the nuclear receptor superfamily. It has been shown that an association exists between Proline 12 alanine (Pro12Ala) polymorphism of PPAR-GAMMA2 (PPAR-γ2) gene and increased risk of type 2 diabetes mellitus (T2DM) in different populations. Therefore, the present study was designed to investigate the association between Pro12Ala polymorphism of PPAR-γ2 gene and T2DM in an Iranian population.
Materials and Methods: Two hundred unrelated people, including 100 healthy controls and 100 diabetic patients were recruited diagnosed based on American Diabetes Association criteria. Blood samples were used for isolation of genomic deoxyribonucleic acid (DNA). Having extracted the genomic DNA from human blood leukocytes by means of High Pure polymerase chain reaction (PCR) Template preparation kit, we carried out polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) on each blood sample. Then, Genomic DNA was digested by BstU-I restriction enzyme. Thereafter, restriction products were analyzed by means of Polyacrylamide gel electrophoresis and stained by Ethidium Bromide.
Results: We found that the frequency of Ala allele in healthy subjects was significantly higher than in diabetic subjects ( P = 0003). Moreover, the genotype frequency of Ala/Ala in healthy subjects was significantly higher than in diabetic subjects ( P < 0.001). However, the genotype frequency of Ala/Pro in diabetic subjects was significantly higher than in healthy subjects ( P < 0.001).
Conclusion: The present study suggests that polymorphism of PPAR-γ2 gene is associated with T2DM. Furthermore, Ala allele is significantly found in non-diabetic individual's Iranian population.
Keywords: Polymorphism, peroxisome proliferator-activated receptor-GAMMA 2 gene, type 2 diabetes mellitus
How to cite this article:
Motavallian A, Andalib S, Vaseghi G, Mirmohammad-Sadeghi H, Amini M. Association between PRO12ALA polymorphism of the PPAR-γ2 gene and type 2 diabetes mellitus in Iranian patients. Indian J Hum Genet 2013;19:239-44 |
How to cite this URL:
Motavallian A, Andalib S, Vaseghi G, Mirmohammad-Sadeghi H, Amini M. Association between PRO12ALA polymorphism of the PPAR-γ2 gene and type 2 diabetes mellitus in Iranian patients. Indian J Hum Genet [serial online] 2013 [cited 2016 May 24];19:239-44. Available from: http://www.ijhg.com/text.asp?2013/19/2/239/116126 |
FNx01Azadeh Motavallian and Sasan Andalib contributed equally to the research
 Introduction Introduction | |  |
Type 2 diabetes mellitus (T2DM) is the most common form of diabetes. It is characterized by a cluster of metabolic dysfunctions and cardiovascular risk factors, such as obesity, insulin resistance, dyslipidemia, atherosclerosis, hypertension, prothrombotic state, and endothelial dysfunction collectively known as the metabolic syndrome. [1] Environmental factors (e.g., obesity and sedentary lifestyles) give rise to T2DM. [2] Moreover, the association between lean first-degree family members of diabetic patients with insulin resistance shows the prominent role of genetic factors in this disease. [3] In spite of intensive research, no specific genes causing T2DM have not yet been conclusively identified. Recent findings have shown that polymorphism of some genes can influence the risk of T2DM. To illustrate, one of these is Peroxisome proliferator-activated receptor-GAMMA (PPAR-γ) gene polymorphism. PPARs have been identified to be ligand-activated transcription factors that belong to the nuclear receptor superfamily. [4] There is evidence that PPAR-γ receptor plays a part in control of blood pressure, insulin sensitivity, and glucose homeostasis. [5] PPAR-α and PPAR-β have shown distinct tissue distribution. [1] There are some data confirming the expression of PPAR-γ in human peripheral blood mononuclear cells. [6],[7] However, PPAR-γ, which has dominantly been found in adipose tissue, serves a significant role in glucose homeostasis, regulation of lipid, and adipocyte differentiation. [8] In spite of the fact that the association between Pro12Al polymorphism of the PPAR-γ2 gene with insulin sensitivity in diabetic patients has been reported in some populations, [9],[10] a number of conflicting results have heightened the need for further investigation. [11],[12],[13] Thus, this study was designed to examine whether the association between polymorphism of the PPAR-γ2 gene and T2DM exists in an Iranian population.
 Materials and Methods Materials and Methods | |  |
Two hundred unrelated people with the average age of 51, including 100 healthy controls and 100 patients with T2DM were recruited from Sedighe Tahere research center. T2DM was clinically diagnosed (polyuria, polydipsia and polyphagia were present) and was confirmed by American Diabetes Association criteria. To clarify, T2DM was determined by [1] a fasting plasma glucose level of more than 7.0 mmol/l after a minimum fast of 12 h or [2] a 2-h post-glucose level (2-h oral glucose tolerance test [OGTT]) of more than 11.1 mmol/l. Impaired glucose tolerance (IGT) was determined by a fasting plasma glucose level of between 5.6 and 7.0 mmol/l or a 2-h OGTT of between 7.8 and 11.1 mmol/l. Normo-glycemic subjects were diagnosed by a fasting glycemia of less than 5.6 mmol/l or a 2-h glucose of less than 7.8 mmol/l. More clinical and biochemical characteristics of the participants are summarized in [Table 1]. Cholesterol, triglycerides, High-density lipoprotein and plasma glucose were measured by standard enzymatic assays. Low-density lipoprotein cholesterol was derived using Friedewald equation. [14] Hemoglobin A1c was measured by means of ion-exchange high performance liquid chromatography with the normal reference range of 4.1-6.4%. Additionally, we applied exclusion criteria as follows: Individuals with IGT and positive family history of T2DM were excluded from the study due to the production of misleading results in analyses of the control group (CG). Individuals with previous diagnosis of blood disease, impaired renal and hepatic function and those receiving pharmacological treatment for T2DM, hypercholesterolemia, or hypertension were also excluded from the study in order to eliminate interference in biological variables. All participants gave a written consent before their enrolment for the study. The study protocol was approved by the Ethics Committee of Isfahan Medical University Research Center.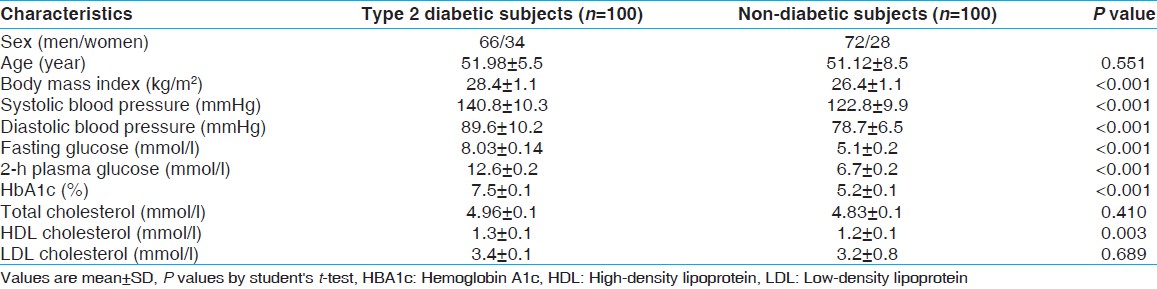 | Table 1: Clinical and biochemical characteristics of type 2 diabetic and non-diabetic subjects
Click here to view |
Obtained blood samples were used for isolation of genomic deoxyribonucleic acid (DNA). Having extracted the genomic DNA from human blood leukocytes by means of High Pure polymerase chain reaction (PCR) Template preparation kit (Roche, Germany), we carried out PCR-RFLP on each sample containing genomic DNA of the patients or controls for detecting PPAR-γ2 gene polymorphism.
Forward primer 5'-CCAATTCAAGCCCAGTCCTTTC-3', and reverse primer 5'-CAGTGAAGGAATCGCTTTCCG-3'. Using BioRad thermal cycler (My Cycler, Germany), each PCR was performed in a volume of 50 μl, including 100 ng of genomic DNA, 10 pmol of each primer, 0.8 mM of deoxynucleotide triphosphates (BIORON), and 0.5 U of Taq DNA polymerase in the reaction buffer (50 mmol/l of KCl, 2.8 mmol/l of MgCl 2 , 10 mmol/l of Tris-HCl, pH 9.0 (BIORON)). For warming up, initial denaturation at 94°C for 3 min was carried out. Thereafter, denaturation at 94°C for 1 min, annealing at 55°C for 1 min, and extension at 72°C for 1 min were performed for 30 cycles. Then it was followed by a post-extension at 72°C for 10 min. In order to confirm the PCR amplification, gel electrophoresis (8% polyacrylamide gel and Ethidium bromide staining) was carried out [Figure 1]. Then, Amplified products (244 bp) were digested by restriction endonuclease BstU-I (Fermentas, Poland) at 37°C for 1 h. In order to analyze the restriction products, electrophoresis was performed using an 8% polyacrylamide gel and Ethidium bromide staining [Figure 2].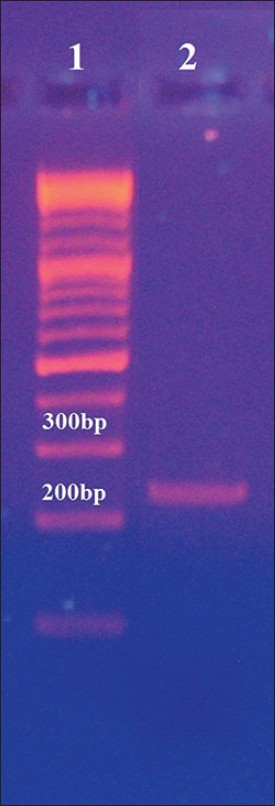 | Figure 1: Confirmation of polymerase chain reaction amplification using gel electrophoresis. Lane 1: 100 bp deoxyribonucleic acid ladder; Lane 2: PCR products from peroxisome proliferator-activated receptor-GAMMA2 gene using the forward and reverse primers mentioned in the method section. Extracted DNA from peripheral blood was used as template
Click here to view |
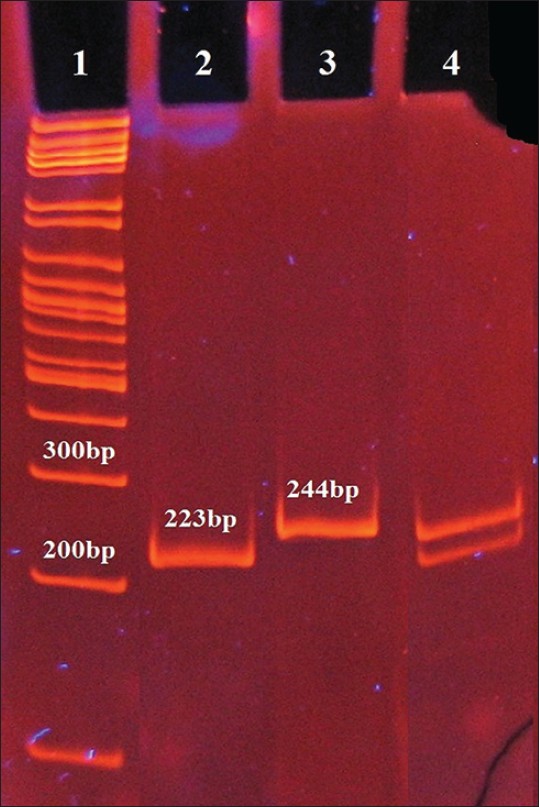 | Figure 2: Polymerase chain reaction-RFLP detection of the Pro12Ala polymorphism of peroxisome proliferator - activated receptor - GAMMA2 gene using gel electrophoresis. Lane 1: 100 bp deoxyribonucleic acid ladder; lane 2: Ala12 homozygotes; lane 3: Pro12 homozygotes; lane 4: Pro12Ala heterozygotes. Extracted DNA from peripheral blood was used as template
Click here to view |
Statistical analyses were performed using the SPSS statistical software package (Version 17.0). More precisely, data were analyzed by Student's t-test and Chi-square test. P < 0.05 was considered significant. Using the χ2 results, the test for Hardy-Weinberg equilibrium and comparison of genotype and allele frequencies in the diabetic and non-diabetic subjects was carried out.
 Results Results | |  |
PCR amplification of PPAR-γ2 gene produced a 244 bp DNA band [Figure 1]. Digestion of this product with BstU-I gave different patterns as follows: Pro12 homozygotes, Ala12 homozygotes, and heterozygotes were shown by one band (244 bp), two fragments (223 and 21 bp), and three fragments (244, 223 and 21 bp), respectively [Figure 2]. What should be noted is that 21 bp fragments were not visualized in the gel.
[Table 2] illustrates the genotype frequency of PPAR-γ2 gene. As can be shown, the genotype frequency of Ala/Ala in healthy subjects was significantly higher than in diabetic subjects (P < 0.001). However, the genotype frequency of Ala/Pro genotype in diabetic subjects was significantly higher than in healthy subjects (P < 0.001). As an aside, in healthy subjects, the genotype frequency of Ala/Ala (0.52) was significantly higher than that of Ala/Pro (0.36) and Pro/Pro (0.12) (P < 0.001). In diabetic subjects, however, the genotype frequency of Ala/Pro (0.56) was significantly higher than that of Ala/Ala (0.2) and Pro/Pro (0.24) (P < 0.001). The frequency of Ala12 carriers (Pro12Ala and Ala12Ala genotypes) in T2DM group (0.76) and CG (0.8) was almost equal.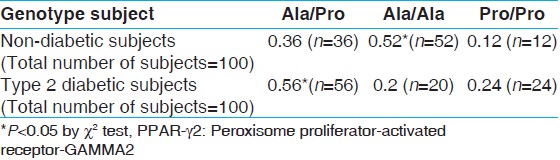 | Table 2: Genotype frequency of PPAR-γ 2 gene (Pro/Pro (homozygous for Pro12 allele), Pro/Ala (heterozygous for Pro12 and Ala12 alleles), Ala/Ala, (homozygous for Ala12 allele)) in type 2 diabetic and non-diabetic subjects
Click here to view |
[Table 3] provides a breakdown pertaining to gene frequency of PPAR-γ2 gene. As can be decidedly noted, the frequency of Ala allele in healthy subjects was significantly higher than in diabetic subjects (P = 0003). As an apart, the frequency of Ala allele in healthy subjects was significantly high, in comparison with that of Pro Allele (P = 0.002). However, in diabetic subjects, the difference between the frequency of Ala allele and Pro allele was not statistically significant.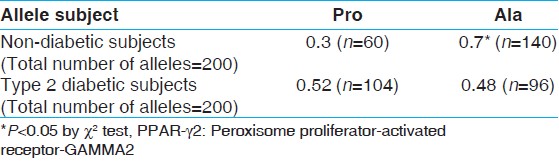 | Table 3: Gene frequency of the PPARγ 2 in type 2 diabetic and non-diabetic subjects note
Click here to view |
Finally, observed genotype frequencies of the Pro12Ala polymorphism of PPAR-γ2 in diabetic and non-diabetic subjects were in accordance with the Hardy-Weinberg equilibrium (data not shown).
 Discussion Discussion | |  |
A large and growing body of literature has investigated the relationship between Pro12Ala polymorphism of PPAR-γ2 gene and various disorders such as T2DM, [15],[16],[17] insulin sensitivity, [18] obesity, [19] cancer, [20] cardiovascular disease [21] and Alzheimer's disease. [22] In as much as there are a number of disagreements on association between Pro12Ala polymorphism of the PPAR-γ2 gene and T2DM, this study set out to determine aforesaid association in an Iranian population.
Our findings were consistent with those of Scacchi et al. who found a protective role of Ala frequency against T2DM prevalence in an Italian population. [8] Tavares et al. showed that carriers of Ala12 allele of the PPAR-γ2 gene were more sensitive to insulin resistance when compared to Pro12 carriers in a Brazilian population. [10] The protection against diabetic nephropathy in Brazilian diabetics caused by presence of Ala allele was reported elsewhere. [23] It was demonstrated that Ala allele brought about lower development of T2DM in Caucasians. [24] Furthermore, our findings were in accord with those of Mori et al. in which they found Ala variant of PPAR-γ was associated with a reduced risk for the development of diabetes in Japanese subjects. [16] Nevertheless, Maciej et al. did not confirm the influence of Pro allele upon increased risk of the development of T2DM. [25] An association was also reported between increased risk of T2DM and Pro allele of PPAR-γ2 gene in a Russian population. [26]
Moreover, present study showed a significant association between Pro12Ala polymorphism of PPAR-γ2 gene with T2DM. We also found that Ala/Ala genotype was significantly present in non-diabetic subjects. Present study produced results, which corroborate the findings of Hara et al. showing the association between Pro12Ala polymorphism of PPAR-γ gene and T2DM in a Japanese population. [27] Another study on Korean subjects showed that PPAR-γ2 Pro12Ala polymorphism was protective against ischemic stroke with T2DM. [28] Horiki et al. demonstrated a correlation between Pro12Ala polymorphism of PPAR-γ and genetic susceptibility to T2DM and hypertension in a Japanese population. [9] The association between PPAR-γ and T2DM in Chinese diabetics was reported elsewhere. [29] A strong relationship between Pro12Ala polymorphism of PPAR-γ2 and T2DM was demonstrated in Sikhs living in northern states of India. [30] However, on the contrary, lack of association between Pro12Ala variant and T2DM was shown in a south Indian population. [31] Furthermore, the study carried out by Stefanski et al. did not detect any evidence for association of Pro12Ala PPAR-γ2 variant with insulin resistance in Caucasian population. [11] It should be mentioned that our findings did not support a preceding Iranian study, which reported no significant association between Pro12Ala polymorphism of PPAR-γ2 and T2DM. [32] Finally, it is somewhat surprising that our study was the first one in Iran that demonstrated an association between polymorphism of PPAR-γ2 and T2DM. It should, however, be noted that lack of association between polymorphism of PPAR-γ2 and T2DM in Northern provinces of Iran was reported in a preceding Iranian study. Taking into an account of these conflicting results, some caveats need to be noted. A small sample was chosen in this study owing to the expected difficulty of obtaining blood samples. In fact, the samples were regionally representative of center of Iran and would naturally tend to miss people who were in marginal regions. Additionally, it appears that controversial findings may be due to population admixture of studied participants. Hence, a cross-national research with larger sample size would help us to better understand the precise effect of Pro12Ala polymorphism of PPAR-γ2 gene upon T2DM risk.
 References References | |  |
| 1. | Gross B, Staels B. PPAR agonists: Multimodal drugs for the treatment of type-2 diabetes. Best Pract Res Clin Endocrinol Metab 2007;21:687-710. 
|
| 2. | Gouda HN, Sagoo GS, Harding AH, Yates J, Sandhu MS, Higgins JP. The association between the peroxisome proliferator-activated receptor-gamma2 (PPARG2) Pro12Ala gene variant and type 2 diabetes mellitus: A huge review and meta-analysis. Am J Epidemiol 2010;171:645-55. 
|
| 3. | Rhee EJ, Oh KW, Lee WY, Kim SY, Oh ES, Baek KH, et al. Effects of two common polymorphisms of peroxisome proliferator-activated receptor-gamma gene on metabolic syndrome. Arch Med Res 2006;37:86-94. 
|
| 4. | Guo L, Tabrizchi R. Peroxisome proliferator-activated receptor gamma as a drug target in the pathogenesis of insulin resistance. Pharmacol Ther 2006;111:145-73. 
|
| 5. | Barroso I, Gurnell M, Crowley VE, Agostini M, Schwabe JW, Soos MA, et al. Dominant negative mutations in human PPARgamma associated with severe insulin resistance, diabetes mellitus and hypertension. Nature 1999;402:880-3. 
|
| 6. | Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature 1998;391:79-82. 
|
| 7. | Garcia-Fuentes E, Murri M, Garrido-Sanchez L, Garcia-Serrano S, García-Almeida JM, Moreno-Santos I, et al. PPARgamma expression after a high-fat meal is associated with plasma superoxide dismutase activity in morbidly obese persons. Obesity (Silver Spring) 2010;18:952-8. 
|
| 8. | Scacchi R, Pinto A, Rickards O, Pacella A, De Stefano GF, Cannella C, et al. An analysis of peroxisome proliferator-activated receptor gamma (PPAR-gamma 2) Pro12Ala polymorphism distribution and prevalence of type 2 diabetes mellitus (T2DM) in world populations in relation to dietary habits. Nutr Metab Cardiovasc Dis 2007;17:632-41. 
|
| 9. | Horiki M, Ikegami H, Fujisawa T, Kawabata Y, Ono M, Nishino M, et al. Association of Pro12Ala polymorphism of PPARgamma gene with insulin resistance and related diseases. Diabetes Res Clin Pract 2004;66:S63-7. 
|
| 10. | Tavares V, Hirata RD, Rodrigues AC, Monte O, Salles JE, Scalissi N, et al. Association between Pro12Ala polymorphism of the PPAR-gamma2 gene and insulin sensitivity in Brazilian patients with type-2 diabetes mellitus. Diabetes Obes Metab 2005;7:605-11. 
|
| 11. | Stefanski A, Majkowska L, Ciechanowicz A, Frankow M, Safranow K, Parczewski M, et al. Lack of association between the Pro12Ala polymorphism in PPAR-gamma2 gene and body weight changes, insulin resistance and chronic diabetic complications in obese patients with type 2 diabetes. Arch Med Res 2006;37:736-43. 
|
| 12. | Hamada T, Kotani K, Tsuzaki K, Sano Y, Murata T, Tabata M, et al. Association of Pro12Ala polymorphism in the peroxisome proliferator-activated receptor gamma2 gene with small dense low-density lipoprotein in the general population. Metabolism 2007;56:1345-9. 
|
| 13. | Stumvoll M, Häring H. The peroxisome proliferator-activated receptor-gamma2 Pro12Ala polymorphism. Diabetes 2002;51:2341-7. 
|
| 14. | Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499-502. 
|
| 15. | Altshuler D, Hirschhorn JN, Klannemark M, Lindgren CM, Vohl MC, Nemesh J, et al. The common PPARgamma Pro12Ala polymorphism is associated with decreased risk of type 2 diabetes. Nat Genet 2000;26:76-80. 
|
| 16. | Mori H, Ikegami H, Kawaguchi Y, Seino S, Yokoi N, Takeda J, et al. The Pro12 Ala substitution in PPAR-gamma is associated with resistance to development of diabetes in the general population: Possible involvement in impairment of insulin secretion in individuals with type 2 diabetes. Diabetes 2001;50:891-4. 
|
| 17. | Yen CJ, Beamer BA, Negri C, Silver K, Brown KA, Yarnall DP, et al. Molecular scanning of the human peroxisome proliferator activated receptor gamma (hPPAR gamma) gene in diabetic Caucasians: Identification of a Pro12Ala PPAR gamma 2 missense mutation. Biochem Biophys Res Commun 1997;241:270-4. 
|
| 18. | Deeb SS, Fajas L, Nemoto M, Pihlajamäki J, Mykkänen L, Kuusisto J, et al. A Pro12Ala substitution in PPARgamma2 associated with decreased receptor activity, lower body mass index and improved insulin sensitivity. Nat Genet 1998;20:284-7. 
|
| 19. | Valve R, Sivenius K, Miettinen R, Pihlajamäki J, Rissanen A, Deeb SS, et al. Two polymorphisms in the peroxisome proliferator-activated receptor-gamma gene are associated with severe overweight among obese women. J Clin Endocrinol Metab 1999;84:3708-12. 
|
| 20. | Meirhaeghe A, Amouyel P. Impact of genetic variation of PPARgamma in humans. Mol Genet Metab 2004;83:93-102. 
|
| 21. | Takano H, Komuro I. Roles of peroxisome proliferator-activated receptor gamma in cardiovascular disease. J Diabetes Complications 2002;16:108-14. 
|
| 22. | Sauder S, Kölsch H, Lütjohann D, Schulz A, von Bergmann K, Maier W, et al. Influence of peroxisome proliferator-activated receptor gamma gene polymorphism on 24S-hydroxycholesterol levels in Alzheimer's patients. J Neural Transm 2005;112:1381-9. 
|
| 23. | Caramori ML, Canani LH, Costa LA, Gross JL. The human peroxisome proliferator-activated receptor gamma2 (PPARgamma2) Pro12Ala polymorphism is associated with decreased risk of diabetic nephropathy in patients with type 2 diabetes. Diabetes 2003;52:3010-3. 
|
| 24. | Huguenin GV, Rosa G. The Ala allele in the PPAR-gamma2 gene is associated with reduced risk of type 2 diabetes mellitus in Caucasians and improved insulin sensitivity in overweight subjects. Br J Nutr 2010;104:488-97. 
|
| 25. | Malecki MT, Frey J, Klupa T, Skupien J, Walus M, Mlynarski W, et al. The Pro12Ala polymorphism of PPARgamma2 gene and susceptibility to type 2 diabetes mellitus in a Polish population. Diabetes Res Clin Pract 2003;62:105-11. 
|
| 26. | Chistiakov DA, Potapov VA, Khodirev DS, Shamkhalova MS, Shestakova MV, Nosikov VV. The PPARgamma Pro12Ala variant is associated with insulin sensitivity in Russian normoglycaemic and type 2 diabetic subjects. Diab Vasc Dis Res 2010;7:56-62. 
|
| 27. | Hara K, Okada T, Tobe K, Yasuda K, Mori Y, Kadowaki H, et al. The Pro12Ala polymorphism in PPAR gamma2 may confer resistance to type 2 diabetes. Biochem Biophys Res Commun 2000;271:212-6. 
|
| 28. | Lee BC, Lee HJ, Chung JH. Peroxisome proliferator-activated receptor-gamma2 Pro12Ala polymorphism is associated with reduced risk for ischemic stroke with type 2 diabetes. Neurosci Lett 2006;410:141-5. 
|
| 29. | Hu C, Zhang R, Wang C, Wang J, Ma X, Lu J, et al. PPARG, KCNJ11, CDKAL1, CDKN2A-CDKN2B, IDE-KIF11-HHEX, IGF2BP2 and SLC30A8 are associated with type 2 diabetes in a Chinese population. PLoS One 2009;4:e7643. 
|
| 30. | Sanghera DK, Demirci FY, Been L, Ortega L, Ralhan S, Wander GS, et al. PPARG and ADIPOQ gene polymorphisms increase type 2 diabetes mellitus risk in Asian Indian Sikhs: Pro12Ala still remains as the strongest predictor. Metabolism 2010;59:492-501. 
|
| 31. | Vimaleswaran KS, Radha V, Jayapriya MG, Ghosh S, Majumder PP, Rao MR, et al. Evidence for an association with type 2 diabetes mellitus at the PPARG locus in a South Indian population. Metabolism 2010;59:457-62. 
|
| 32. | Mirzaei H, Akrami SM, Golmohammadi T, Doosti M, Heshmat R, Nakhjavani M, et al. Polymorphism of Pro12Ala in the peroxisome proliferator-activated receptor gamma2 gene in Iranian diabetic and obese subjects. Metab Syndr Relat Disord 2009;7:453-8. 
|
[Figure 1], [Figure 2]
[Table 1], [Table 2], [Table 3]
| This article has been cited by | | 1 |
Probing the Single Nucleotide Polymorphism (Snp) of Swine PPAR Delta Gene |
|
| W.Y. Liu | | Biotechnology(Faisalabad). 2013; 12(4): 183 | | [Pubmed] | [DOI] | |
|
 |
|






