|
 
 |
|
ORIGINAL ARTICLE |
|
|
|
| Year : 2013 | Volume
: 19
| Issue : 4 | Page : 403-407 |
| |
Protein tyrosine phosphatase non-receptor type 22 gene polymorphism C1858T is not associated with leprosy in Azerbaijan, Northwest Iran
Mohammad Reza Aliparasti1, Shohreh Almasi2, Jafar Majidi2, Fatemeh Zamani2, Ali Reza Khoramifar3, Ali Reza Farshi Azari3
1 Drug Applied Research Center; Immunology Research Center; Department of Immunology, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
2 Immunology Research Center; Department of Immunology, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
3 Bababaghi Hospital, Tabriz University of Medical Sciences, Tabriz, Iran
| Date of Web Publication | 4-Jan-2014 |
Correspondence Address:
Shohreh Almasi
Immunology Research Center, Department of Immunology, Faculty of Medicine, Tabriz University of Medical Sciences, Golgasht Street, Daneshgah Street, Tabriz
Iran
 Source of Support: None, Conflict of Interest: None  | 2 |
DOI: 10.4103/0971-6866.124365

 Abstract Abstract | | |
Background: Leprosy (Hansen's disease) is a human chronic granulomatous infectious disease caused by Mycobacterium leprae. Several types of study support a role for host genetics in susceptibility to leprosy. The protein tyrosine phosphatase non-receptor type 22 (PTPN22) gene encodes an intracellular lymphoid protein tyrosine phosphatase that has been shown to play a negative regulatory role in T-cell activation.
Aims: The aim of the present study was to find out associating the PTPN22 C1858T (R620W) polymorphism and leprosy in the Azeri population from Northwest Iran.
Materials and Methods: A total of 153 treated leprosy patients and 197 healthy and ethnic matched controls entered this study. We used restriction fragment length polymorphism method to type PTPN22 C1858T polymorphism.
Results: There was no significant difference in distribution of genotype and allele frequencies of PTPN22 C1858T polymorphism between leprosy patients and controls (P = 0.641 and 0.645; respectively). Moreover, there was no significant association between different clinical findings (karnofsky performance status score, clinical forms and manifestations of leprosy) and PTPN22 C1858T polymorphism. Data showed a low frequency of the minor (T) allele by 2.3% in leprosy and 1.5% in healthy individuals.
Conclusions: The PTPN22 C1858T (R620W) is not relevant in susceptibility to leprosy in the Azeri population of Northwest Iran.
Keywords: Gene polymorphism, leprosy, protein tyrosine phosphatase non-receptor type 22
How to cite this article:
Aliparasti MR, Almasi S, Majidi J, Zamani F, Khoramifar AR, Azari AF. Protein tyrosine phosphatase non-receptor type 22 gene polymorphism C1858T is not associated with leprosy in Azerbaijan, Northwest Iran. Indian J Hum Genet 2013;19:403-7 |
How to cite this URL:
Aliparasti MR, Almasi S, Majidi J, Zamani F, Khoramifar AR, Azari AF. Protein tyrosine phosphatase non-receptor type 22 gene polymorphism C1858T is not associated with leprosy in Azerbaijan, Northwest Iran. Indian J Hum Genet [serial online] 2013 [cited 2016 May 24];19:403-7. Available from: http://www.ijhg.com/text.asp?2013/19/4/403/124365 |
 Introduction Introduction | |  |
Leprosy, also known as Hansen's disease, is a chronic granulomatous infectious disease caused by the obligate intracellular organism Mycobacterium leprae, that has a liking for the skin and nerves. [1],[2] Leprosy is still an important health problem world-wide, with the highest incidences in Asia, Africa and Latin America. [3] Infection is necessary for the onset of disease, but only a few infections lead to clinically recognizable lesions. [4] Human genetic factors influence the acquisition of leprosy and the clinical course of the disease. [5],[6],[7] Damage development in leprosy individuals is normally caused by the host immune responses driven to mycobacterial elimination. [8] Ridley and Jopling have classified clinical leprosy uses histopathological and clinical features and the bacteriological index as well, into five types: Tuberculoid leprosy (TT) and lepromatous leprosy (LL) with three borderline groups, borderline tuberculoid (BT), borderline borderline (BB) and borderline lepromatous (BL). [9] Tuberculoid leprosy patients have a fairly successful M. leprae specific cell-mediated immune response. Their lesions are characterized by epithelioid cell granulomas, participation of lymphocytes (mainly of Th1 type) and few if any detectable bacilli. In contrast, in the lepromatous form, the specific cellular immunity against M. leprae is almost absent, [10] with diffuse dermal infiltrates characterized by poorly distinguished young macrophages with a heavy load of bacilli and a few T-cells mainly of the Th2 type. [11] Thus, a protective immune response in leprosy is considered to rely on the cellular arm of the immune system, specifically on the generation of helper T-cells that can activate cells from the monocytic lineage (macrophages, dendritic cells and Schawnn cells, among others) to destroy the bacilli that they harbor, along with effector T-cells, giving them capable of killing these infected cells. Therefore, T-cell activation is a crucial step in leprosy immunity. [12]
Several host genes that modulate the immune response to M. leprae infection have been suggested to influence the acquisition of leprosy as well as its clinical course. [8],[13]
The protein tyrosine phosphatase non-receptor type 22 (PTPN22) gene, located on chromosome 1p13.3-p13.1 and encodes a lymphoid protein tyrosine phosphatase (LYP). [14] LYP, which is specifically expressed in lymphocytes, is important in the negative control of T-cell activation, by dephosphorylating the kinases which recognized to be important in T-cell signaling, [15],[16],[17],[18] as well as, binding to the adaptor molecule Grb2 (growth factor receptor-bound protein 2). [19] Also, LYP through the formation of a complex with C-terminal Src Kinas (CSK) suppresses the downstream mediators of T-cell receptor signaling and downregulates the activation of T-cells. [14],[20]
Attia et al., have reported the circulating regulatory T-cells vary in different clinical forms of leprosy. [21] Moreover, a recent study has shown the PTPN22 alters the development of T regulatory cells in the thymus. [22]
Several studies have shown that a functional mission PTPN22 C1858T (R620W) polymorphism confer susceptibility to several autoimmune diseases. [23],[24],[25],[26],[27]
In two separate studies, Lamsyah and others and Gomez et al., remarked a significant association of the PTPN22 C1858T polymorphism with pulmonary tuberculosis (PTB) (which is caused by Mycobacterium tuberculosis) in a Moroccan population, [28] and Columbian population, [29] respectively.
Owing to the pivotal role of T-cell activation in immunity against leprosy, as well inhibitory effects of PTPN22 on the T-cell activation, for the first time in the world, we explored whether the polymorphism in the C1858T region of PTPN22 gene (R620W) was associated with susceptibility to the disease in the Azeri population of Northwest Iran.
 Materials and Methods Materials and Methods | |  |
Patients and controls
A total of 153 unrelated inactive treated leprosy patients which consists of 66 females and 87 males who were residing in Bababaghi Hospice in Tabriz, enrolled in this case-control study, from September 2008 to September 2010. The control group consisted of 197 unrelated healthy volunteers (98 females and 99 males) who were ethnically matched to the patients. Diagnosis of leprosy was established according to World Health Organization criteria. With classification according to the Ridley-Jopling system, there were 108 LL, 37 TT, 6 BB and 2 BT leprosy patients in this study. Their performance status was quantified by karnofsky performance status (KPS) scale. [30] Performance was graded as mild (KPS 80-100), moderate (KPS 50-70) and severe (KPS 0-40). Furthermore, we determined other clinical variables such as age, sex, onset age of the disease, disease duration and clinical manifestation of disease. Ethnically matched healthy subjects with no clinical evidence or family history of autoimmune diseases, cancers, asthma and chronic infectious diseases such as leprosy were recruited in the Tabriz blood transfusion service. The project was approved by the Ethics Committee of Tabriz University of Medical Sciences and informed consent was obtained from all patients and healthy subjects. All joining individuals in this study were belonged to the Azeri ethnic group of Northwest Iran.
Blood sampling
Ethylenediaminetetraacetic acid-added whole blood was collected from leprosy patients and controls.
Deoxyribonucleic acid (DNA) extraction
Genomic DNA was extracted from peripheral blood leukocytes by the proteinase K method.
Polymorphism typing of PTPN22 C1858T
PTPN22 C1858T polymorphism was genotyped by polymerase chain reaction (PCR)-restriction fragment length polymorphism. The forward and reverse primers were, respectively, 5′- ACTGATAATGTTGCTTCAACGG -3′ and 5′- TCACCAGCTTCCTCAACCAC -3′.
25 μl of PCR reaction mixture consisting of 375 ng of genomic DNA, 200 μmol/L dNTPs, 1.75 mM MgCl 2 , 1 × PCR buffer, 1 units of Taq DNA polymerase (Bioflex, Japan), 5 pmol of each test primer were employed. PCR reaction was carried out with the Mastercycler (peQlab, Germany) machine. Samples were initially denatured for 2 min at 94°C followed by 30 cycles of 94°C for 30 s, 30 s at 60°C and 90 s at 70°C. A 218-bp fragment containing the C1858T single nucleotide polymorphism (R620W) within the coding region of the PTPN22 gene was amplified. The products were checked on 2% agarose gel (Invitrogen, USA) containing ethidium bromide. Amplified products were digested using 10 units of RsaI (Thermo Scientific, Lithuania) per reaction overnight at 37°C. The mutant 1858T allele cannot be digested and yields one fragment of 218 bp, while the 1858C allele creates restriction site producing two fragments of 172 bp and 46 bp. Restriction patterns were observed by 3% agarose gel (Invitrogen, USA) electrophoresis with ethidium bromide staining.
Statistical analysis
Data were analyzed by Pearson Chi-squared and Fisher's exact tests when proper. Similarly, genotype frequencies were compared within the patient series, stratified according to disease forms and clinical manifestations of disease using the Chi-square analysis or the Fisher's exact test when appropriate. Association of polymorphisms with age at disease onset, KPS score were determined using non-parametric Kruskal-Wallis test. Statistical calculations were carried out using SPSS (version 16.0, SPSS Inc., Chicago, USA) and Epi Info 2002 (Centers for Disease Control and Prevention, Atlanta, GA USA) statistical software packages. Statistical significance was defined as P < 0.05. Hardy-Weinberg proportions were determined by applying the equation (p 2 + 2pq + q 2 ).
 Results Results | |  |
1858 PTPN22 C/T polymorphism was in Hardy-Weinberg equilibrium in patients and controls. The allele and genotype frequencies in the case and control groups are shown in [Table 1]. As shown in this table, no significant differences were demonstrated for 1858 PTPN22 C/T genotype and allele frequencies in the leprosy patients compared with the control group (P = 0.641 and 0.645; respectively). There were also no significant differences in the 1858 PTPN22 C/T genotype distributions between patients with different clinical forms and manifestations of leprosy (P = 0.757 and P = 0.144, respectively). In addition, there were no significant differences among clinical parameters (including the KPS score and the age of disease onset) and different genotypes of 1858 PTPN22 C/T polymorphisms in patients (P > 0.05).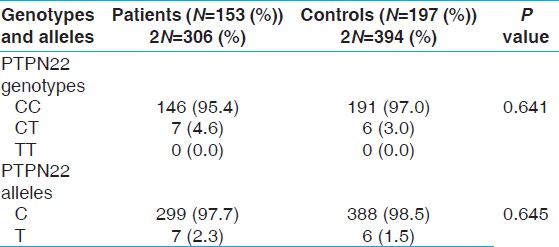 | Table 1: Genotype and allele frequencies of PTPN22 C1858T polymorphism in leprosy patients and controls
Click here to view |
No homozygous TT genotype was detected among leprosy patients or healthy subjects.
 Discussion Discussion | |  |
In our study, we found no relationship between the genotype and allelic frequencies of the PTPN22 C1858T gene polymorphism and vulnerability to leprosy. To the best of our knowledge, there is no investigation on exploring the association between PTPN22 polymorphisms and leprosy.
There are a few studies on evaluating the role of PTPN22 C1858T polymorphism in susceptibility to intracellular bacterial diseases. Two separate studies, in a Moroccan population [28] and Columbian population, [29] have reported the significant association of PTPN22 C1858T polymorphism with pulmonary tuberculosis (PTB). In these investigations, a potential protective role of the T allele of PTPN22 in PTB has been suggested. The PTPN22 1858C >T SNP changes the amino acid at position 620 from an arginine (R) to a tryptophan (W) and disrupts the interaction between LYP and CSK, avoiding forming the complex and therefore, suppressing T-cell activation. In vitro experiments have shown the T allele of PTPN22 binds less efficiently to CSK than the C allele does, suggesting that T-cells expressing the T allele may be hyper responsive and so, individuals carrying this allele may be prone to autoimmunity. [31],[32]
In concordance with our finding, Kouhpayeh et al. found the PTPN22 C1858T is not involved in the susceptibility to PTB in Iranian patients of Southeast Iran. [33] These conflicting results may be a reflection of genetic heterogeneity and potential differences in linkage disequilibrium among various racial populations.
Bravo et al. has reported the PTPN22 C1858T polymorphism is not associated with susceptibility to Brucella More Details melitensis, an intracellular pathogen, which causes the human Brucellosis More Details. [34]
Variation in allele frequency of the PTPN22 C1858T (R620W) has been reported among different ethnic populations. [33] To the best of our knowledge, the highest frequency of the minor allele (T allele) was found to be 15% in Finland. [35] A comprehensive population study showed low frequency of T allele in China (1.43%), [36] besides in some populations such as Azeri of the republic of Azerbaijan, this polymorphism indeed has not been found (T allele frequency of 0.37%). [37] Kouhpayeh et al., found a low frequency of the T allele frequency of 0.8% in PTB and 2.0% in normal individuals, as well as, they did not detect individuals carrying the TT genotype among patients and controls, which shows that PTPN22 is less polymorphic in the Southeast Iranian population. [33] Hence, in line with these observations, in the present study we found similar results (T allele frequency of 2.3% in leprosy and 1.5% in normal individuals) and not seeing the TT genotype carriers among leprosy patients and controls, for the first time in a sample of the Azeri Iranian population. These observations show PTPN22 is fewer polymorphic in our population. The low prevalence of minor allele in our population may account for the lack of association with leprosy. Therefore, further studies among different ethnicity populations are recommended.
 Conclusion Conclusion | |  |
The present study didn't find any association of PTPN22 C1858T (R620W) with susceptibility to leprosy in Azeri patients of Northwest Iran.
 Acknowledgments Acknowledgments | |  |
We would like to thank all leprosy patients and healthy individuals who vulnerably participated in this study.
 References References | |  |
| 1. | Modlin RL. The innate immune response in leprosy. Curr Opin Immunol 2010;22:48-54. 
|
| 2. | Montoya D, Modlin RL. Learning from leprosy: Insight into the human innate immune response. Adv Immunol 2010;105:1-24. 
|
| 3. | Vannberg FO, Chapman SJ, Hill AV. Human genetic susceptibility to intracellular pathogens. Immunol Rev 2011;240:105-16. 
|
| 4. | Quintana-Murci L, Alcaïs A, Abel L, Casanova JL. Immunology in natura: Clinical, epidemiological and evolutionary genetics of infectious diseases. Nat Immunol 2007;8:1165-71. 
|
| 5. | Alcaïs A, Mira M, Casanova JL, Schurr E, Abel L. Genetic dissection of immunity in leprosy. Curr Opin Immunol 2005;17:44-8. 
|
| 6. | Alter A, Grant A, Abel L, Alcaïs A, Schurr E. Leprosy as a genetic disease. Mamm Genome 2011;22:19-31. 
|
| 7. | Cardoso CC, Pereira AC, de Sales Marques C, Moraes MO. Leprosy susceptibility: Genetic variations regulate innate and adaptive immunity, and disease outcome. Future Microbiol 2011;6:533-49. 
|
| 8. | Fitness J, Tosh K, Hill AV. Genetics of susceptibility to leprosy. Genes Immun 2002;3:441-53. 
|
| 9. | Ridley DS, Jopling WH. Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis 1966;34:255-73. 
|
| 10. | Faber WR, Leiker DL, Nengerman IM, Zeijlemaker WP, Schellekens PT. Lymphocyte transformation test in leprosy: Decreased lymphocyte reactivity to Mycobacterium leprae in lepromatous leprosy, with no evidence for a generalized impairment. Infect Immun 1978;22:649-56. 
|
| 11. | Yamamura M, Uyemura K, Deans RJ, Weinberg K, Rea TH, Bloom BR, et al. Defining protective responses to pathogens: Cytokine profiles in leprosy lesions. Science 1991;254:277-9. 
|
| 12. | Murray RA, Siddiqui MR, Mendillo M, Krahenbuhl J, Kaplan G. Mycobacterium leprae inhibits dendritic cell activation and maturation. J Immunol 2007;178:338-44. 
|
| 13. | Mira MT. Genetic host resistance and susceptibility to leprosy. Microbes Infect 2006;8:1124-31. 
|
| 14. | Cohen S, Dadi H, Shaoul E, Sharfe N, Roifman CM. Cloning and characterization of a lymphoid-specific, inducible human protein tyrosine phosphatase, LYP. Blood 1999;93:2013-24. 
|
| 15. | Mustelin T, Brockdorff J, Rudbeck L, Gjörloff-Wingren A, Han S, Wang X, et al. The next wave: Protein tyrosine phosphatases enter T cell antigen receptor signalling. Cell Signal 1999;11:637-50. 
|
| 16. | Hermiston ML, Xu Z, Majeti R, Weiss A. Reciprocal regulation of lymphocyte activation by tyrosine kinases and phosphatases. J Clin Invest 2002;109:9-14. 
|
| 17. | Mustelin T, Abraham RT, Rudd CE, Alonso A, Merlo JJ. Protein tyrosine phosphorylation in T cell signaling. Front Biosci 2002;7:d918-69. 
|
| 18. | Veillette A, Latour S, Davidson D. Negative regulation of immunoreceptor signaling. Annu Rev Immunol 2002;20:669-707. 
|
| 19. | Hill RJ, Zozulya S, Lu YL, Ward K, Gishizky M, Jallal B. The lymphoid protein tyrosine phosphatase LYP interacts with the adaptor molecule Grb2 and functions as a negative regulator of T-cell activation. Exp Hematol 2002;30:237-44. 
|
| 20. | Cloutier JF, Veillette A. Cooperative inhibition of T-cell antigen receptor signaling by a complex between a kinase and a phosphatase. J Exp Med 1999;189:111-21. 
|
| 21. | Attia EA, Abdallah M, Saad AA, Afifi A, El Tabbakh A, El-Shennawy D, et al. Circulating CD4 + CD25 high FoxP3 + T cells vary in different clinical forms of leprosy. Int J Dermatol 2010;49:1152-8. 
|
| 22. | Maine CJ, Hamilton-Williams EE, Cheung J, Stanford SM, Bottini N, Wicker LS, et al. PTPN22 alters the development of regulatory T cells in the thymus. J Immunol 2012;188:5267-75. 
|
| 23. | Gomez LM, Anaya JM, Gonzalez CI, Pineda-Tamayo R, Otero W, Arango A, et al. PTPN22 C1858T polymorphism in Colombian patients with autoimmune diseases. Genes Immun 2005;6:628-31. 
|
| 24. | Chelala C, Duchatelet S, Joffret ML, Bergholdt R, Dubois-Laforgue D, Ghandil P, et al. PTPN22 R620W functional variant in type 1 diabetes and autoimmunity related traits. Diabetes 2007;56:522-6. 
|
| 25. | Lee YH, Rho YH, Choi SJ, Ji JD, Song GG, Nath SK, et al. The PTPN22 C1858T functional polymorphism and autoimmune diseases - A meta-analysis. Rheumatology (Oxford) 2007;46:49-56. 
|
| 26. | Douroudis K, Prans E, Haller K, Nemvalts V, Rajasalu T, Tillmann V, et al. Protein tyrosine phosphatase non-receptor type 22 gene variants at position 1858 are associated with type 1 and type 2 diabetes in Estonian population. Tissue Antigens 2008;72:425-30. 
|
| 27. | Zhebrun D, Kudryashova Y, Babenko A, Maslyansky A, Kunitskaya N, Popcova D, et al. Association of PTPN22 1858T/T genotype with type 1 diabetes, Graves' disease but not with rheumatoid arthritis in Russian population. Aging (Albany NY) 2011;3:368-73. 
|
| 28. | Lamsyah H, Rueda B, Baassi L, Elaouad R, Bottini N, Sadki K, et al. Association of PTPN22 gene functional variants with development of pulmonary tuberculosis in Moroccan population. Tissue Antigens 2009;74:228-32. 
|
| 29. | Gomez LM, Anaya JM, Martin J. Genetic influence of PTPN22 R620W polymorphism in tuberculosis. Hum Immunol 2005;66:1242-7. 
|
| 30. | Mor V, Laliberte L, Morris JN, Wiemann M. The karnofsky performance status scale. An examination of its reliability and validity in a research setting. Cancer 1984;53:2002-7. 
|
| 31. | Bottini N, Musumeci L, Alonso A, Rahmouni S, Nika K, Rostamkhani M, et al. A functional variant of lymphoid tyrosine phosphatase is associated with type I diabetes. Nat Genet 2004;36:337-8. 
|
| 32. | Begovich AB, Carlton VE, Honigberg LA, Schrodi SJ, Chokkalingam AP, Alexander HC, et al. A missense single-nucleotide polymorphism in a gene encoding a protein tyrosine phosphatase (PTPN22) is associated with rheumatoid arthritis. Am J Hum Genet 2004;75:330-7. 
|
| 33. | Kouhpayeh HR, Hashemi M, Hashemi SA, Moazeni-Roodi A, Naderi M, Sharifi-Mood B, et al. R620W functional polymorphism of protein tyrosine phosphatase non-receptor type 22 is not associated with pulmonary tuberculosis in Zahedan, southeast Iran. Genet Mol Res 2012;11:1075-81. 
|
| 34. | Bravo MJ, Colmenero JD, Queipo-Ortuño MI, Morata P, Orozco G, Martin J, et al. PTPN22 C1858T polymorphism and human brucellosis. Scand J Infect Dis 2009;41:109-12. 
|
| 35. | Seldin MF, Shigeta R, Laiho K, Li H, Saila H, Savolainen A, et al. Finnish case-control and family studies support PTPN22 R620W polymorphism as a risk factor in rheumatoid arthritis, but suggest only minimal or no effect in juvenile idiopathic arthritis. Genes Immun 2005;6:720-2. 
|
| 36. | Zhang ZH, Chen F, Zhang XL, Jin Y, Bai J, Fu SB. PTPN22 allele polymorphisms in 15 Chinese populations. Int J Immunogenet 2008;35:433-7. 
|
| 37. | Cinek O, Hradsky O, Ahmedov G, Slavcev A, Kolouskova S, Kulich M, et al. No independent role of the -1123 G>C and + 2740 A>G variants in the association of PTPN22 with type 1 diabetes and juvenile idiopathic arthritis in two Caucasian populations. Diabetes Res Clin Pract 2007;76:297-303. 
|
[Table 1]
| This article has been cited by | | 1 |
Analysis of PTPN22 C1858T gene polymorphism in cases with type 1 diabetes of Azerbaijan, Northwest Iran |
|
| Shohreh Almasi,Mohammad Reza Aliparasti,Leili Yazdchi-Marandi,Akbar Aliasgarzadeh,Amirbabak Sioofy-Khojine,Adel Mesri,Fatemeh Zamani | | Cellular Immunology. 2014; 292(1-2): 14 | | [Pubmed] | [DOI] | |
|
 |
|






