|
 
 |
|
ORIGINAL ARTICLE |
|
|
|
| Year : 2014 | Volume
: 20
| Issue : 2 | Page : 155-159 |
| |
Methylenetetrahydrofolate reductase C677T variant in Indian children with craniosynostosis: Its role in the pathogenesis, risk of craniosynostosis
Rajeev Kumar Pandey, Abid Ali, Amit Singh, Sukanya Gayan, Minu Bajpai
Department of Pediatric Surgery, All India Institute of Medical Sciences, New Delhi, India
| Date of Web Publication | 14-Oct-2014 |
Correspondence Address:
Minu Bajpai
Department of Pediatric Surgery, All India Institute of Medical Sciences, New Delhi - 110 029
India
 Source of Support: None, Conflict of Interest: None
DOI: 10.4103/0971-6866.142882

 Abstract Abstract | | |
Background: 677C to T allele in the 5, 10-methylenetetrahydrofolate reductase (MTHFR) gene has been implicated in the etiology of various syndromes and nonsyndromic diseases but till date no direct studies have been reported with craniosynostosis.
Objectives: The aim was to study the family-based association of MTHFR polymorphism in different categories of craniosynostosis patients.
Materials and Methods: This was a cross-sectional study in which 30 patients classified as Apert syndrome, Pfeiffr syndrome and nonsyndromic craniosynostosis patients with their family were recruited. A sample of 3 ml intravenous blood was taken from patients and from their family members (father and mother) in ethylenediaminetetraacetic acid-anticoagulated vacutainer for the purpose of the study. Genomic DNA was extracted from peripheral blood lymphocytes by phenol chloroform extraction method. Primers for MTHFR gene were designed. The polymerase chain reaction was carried out. After successful amplification, a small aliquot (5 μl) of the MTHFR reaction mixture was treated with 1 units of Hinf I restriction enzyme (NEB). Results were obtained and compiled.
Results: A total of 30 patients/participants with craniosynostosis of Indian descent and their parents formed the study group. The genotyping did not confirm an association between the MTHFR 677C to T polymorphism and between different categories of craniosynostosis. When comparing the offspring of mothers statistically significant differences were found.
Conclusion: C667T polymorphism of the MTHFR gene is unlikely to play a role in the pathogenesis of craniosynostosis though maternal MTHFR C677T polymorphism may be a genetic risk factor.
Keywords: Craniosynostosis, gene, methylenetetrahydrofolate reductase polymorphism
How to cite this article:
Pandey RK, Ali A, Singh A, Gayan S, Bajpai M. Methylenetetrahydrofolate reductase C677T variant in Indian children with craniosynostosis: Its role in the pathogenesis, risk of craniosynostosis. Indian J Hum Genet 2014;20:155-9 |
How to cite this URL:
Pandey RK, Ali A, Singh A, Gayan S, Bajpai M. Methylenetetrahydrofolate reductase C677T variant in Indian children with craniosynostosis: Its role in the pathogenesis, risk of craniosynostosis. Indian J Hum Genet [serial online] 2014 [cited 2016 Aug 23];20:155-9. Available from: http://www.ijhg.com/text.asp?2014/20/2/155/142882 |
 Introduction Introduction | |  |
Methylenetetrahydrofolate reductase (MTHFR) is a critical enzyme in folate metabolism. Folate is essential to the carbon transfer necessary for DNA synthesis, cell division, and tissue growth. [1] MTHFR is actively involved in DNA methylation, DNA synthesis, and DNA repair. The MTHFR gene is located at chromosome 1p36.3 and is 2.2 kb in length with a total of 11 exons. Within the MTHFR gene a common C to T polymorphism exists in exon 4 at position 677, it is a point mutation that converts a cytosine (C) to a thymine (T), resulting in an amino acid substitution of alanine to valine. The T variant codes for a thermolabile enzyme leading to an activity of 65% in the heterozygous state (CT) and ~30% in the homozygous state (TT), respectively. [2] Several association studies have been conducted to assess whether an association exists between the polymorphism in the MTHFR gene (MIM 607093). [3] MTHFR gene mutation has been related to many diseases including colon cancer, leukemia, vascular disease, depression, schizophrenia, migraine with aura, glaucoma, Down syndrome, and neural tube defects.
Skull development is a complex process that involves on going interaction between the bones of the skull and cranial soft tissues. The cranial vault is comprised of intramembranous bones joined by the sutures of dense fibrous tissue that accommodate the growing brain. Bone is added at these sutures during growth, and the skull eventually ossifies completely. Craniosynostosis is the premature closure of one or more of the cranial vault sutures. It is a common congenital anomaly. Most of the craniosynostosis belongs to nonsyndromic group without major associated malformations. [4] Well-known mutations in the fibroblast growth factor receptor-2 (FGFR2), TWIST1, FREM1, LRIT3, EFNA4 and RUNX2 have not shown constant results with different ethnic population with nonsyndromic craniosynostosis. [5],[6],[7],[8],[9],[10],[11],[12] Around ~20% of craniosynostosis cases are syndromic, occurring with one or more additional major malformations caused by single-gene mutations in one of at least eight genes (FGFR1, FGFR2, FGFR3, TWIST1, EFNB1, POR, MSX2 and RAB23), involving primarily the coronal sutures. [13],[14],[15] Recently, Rajagopalan et al., have reported that Common foliate gene variant, MTHFR C677T, is associated with brain structure in two independent cohorts of people with mild cognitive impairment. They found that a very commonly carried variant in the MTHFR gene, which is associated with high homocysteine levels in the blood, is significantly associated with brain structure variation, in particular with lower regional brain volumes, [16] As per our knowledge, none of the studies had solely examined the association of MTHFR gene C to T polymorphism in craniosynostosis patients in any part of the world. As a first step for a comprehensive genetic study on craniosynostosis, we performed a family-based association study of the MTHFR polymorphism. This family-based association approach has the advantage that it avoids possible ethnic stratification that may affect the conventional case-control design.
Objectives
To study the family-based association of the MTHFR polymorphism in different categories of craniosynostosis patients.
 Materials and Methods Materials and Methods | |  |
This was a cross-sectional study in which 30 patients classified as Apert syndrome, Pfeiffr syndrome and nonsyndromic craniosynostosis patients with their family were recruited after obtaining clearance from the Institutional Ethics Committee. In clinically suspected cases of craniosynostosis an X-ray or a CT scan of the child's skull was done. A complete prenatal and birth history of the child, including family history of craniosynostosis or other craniofacial abnormalities was recorded. Three milliliter intravenous blood was collected from patients and from their family members (father and mother) in ethylenediaminetetraacetic acid-anticoagulated vacutainer for the purpose of the study after their written informed consent. Children with primary microcephaly (secondary craniosynostosis) and postural plagiocephaly and those with any chronic diseases or associated syndromic disorders, parents and relatives not giving consent were excluded from the study. Genomic DNA was extracted from peripheral blood lymphocytes by phenol chloroform extraction method. [17],[18] Primers for MTHFR gene were designed as described by Reutter et al. [19],[20] and custom-synthesized primers (Sigma Aldrich Chemicals Pvt., Ltd., Bengaluru, India). Polymerase chain reaction (PCR) for each sample was performed in 0.2 mL, thin-walled tubes using 20 μg of DNA, 2-5 pm of each primer, 200 mM dinucleotide triphosphates, ×10 PCR buffer, 1.5 mM MgCl2, and 0.5 units of DyNAzyme II DNA polymerase (Thermo Scientific). The PCR reaction was carried out in a T-100 DNA Engine (Bio-Rad, Hercules, CA, USA) thermal cyclers under the following conditions initial - denaturation - 94°C for 8 min, denaturation - 94°C for 1 min, annealing - 63°C for 1 min, extension - 72°C for 1 min, final extension - 72°C for 7 min 4°C, forever repeated for 40 cycles. The primer sequences were: Modified primers (F5-TCTTCATCCCTCGCCTTGAAC-3; R5-AGGACGGTGCGGTGAGAGTG-3) according to Frosst et al. (1995). Amplicons sized were verified by gel electrophoresis by running the PCR product on 2% agarose gel with the 100 bp maker [Figure 1]. After successful amplification, a small aliquot (5 μl) of the MTHFR reaction mixture was treated with 1 units of Hinf I restriction enzyme (NEB). | Figure 1: Two percent agarose gel, polymerase chain reaction (PCR) product before Hinf I restriction fragment length polymorphism; Lane 1 - 100 bp ladder; Lane 2 - Blank; Lanes 3, 4, 5, 6, 7 and 8 - PCR product of 315 bp
Click here to view |
Restriction fragment length polymorphism
The amplified PCR products (MTHFR) were subjected to Hinf I restriction enzyme digestion at 37°C for 1 h. The PCR products subjected to enzyme digestion was visualized on 3% agarose gel stained with ethidium bromide. Gel photography was done with bio-red gel doc system. For MTHFR 677, the PCR yielded a 315 bp product, which on digestion with Hinf I produced a 176 bp and 139 bp fragments for TT condition (homozygous polymorphic) and a 315,176 and 139 bp fragments for CT condition (heterozygous polymorphic). An undigested product length of 315 bp was retained by the wild types [Figure 2]. Results were obtained and compiled. | Figure 2: Three percent agarose gel, Hinf I restriction fragment length polymorphism analysis of methylenetetrahydrofolate reductase 677; Lane 1-50 bp ladder; Lanes 2, 3, 4, 6 and 8 - homozygous wild type; Lane 5 and 7 -heterozygous polymorphic
Click here to view |
 Results Results | |  |
The allele and genotype frequencies of the MTHFR of offspring and parents were as tabulated in [Table 1]. These results suggest no significant association of markers with craniosynostosis in the cases. The distribution of MTHFR 677 polymorphisms among mothers and babies. It was observed that for MTHFR C677T, three out of four cases were in the group pertaining to both mother and baby being carriers of polymorphic variant. In two cases, both fathers and mother carried a polymorphic variant to the patient suggesting that maternal MTHFR C677T polymorphism may be a genetic risk factor for craniosynostosis.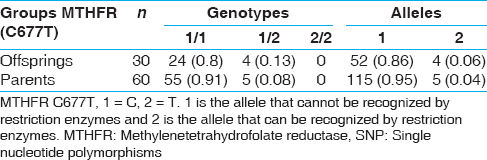 | Table 1: Allele and genotype frequencies of the MTHFR SNP in the craniosynostosis trios
Click here to view |
 Discussion Discussion | |  |
The folate metabolism pathway plays an important role in DNA methylation, DNA synthesis, cell division, and tissue growth, especially in the rapidly developing cells. Thus, a defective folate metabolism could result in an impaired DNA synthesis or DNA methylation involved in the craniosynostosis. Homozygous c. 677C >T + c. 677C >T in the Methylene tetrahydrofolate reductase (MTHFR) gene is associated with higher than normal levels of homocysteine, which may increase risk of thrombosis (MTHFR thermolabile variant thrombophilia). In addition, this mutation/polymorphism is considered a MTHFR thermolabile variant and is associated with hyperhomocysteinemia, increased cardiovascular risk, increased risk of neural tube defects, and increased risk of preeclampsia. Furthermore, mutations in the MTHFR gene may cause MTHFR deficiency/homocystinuria. To the best of our knowledge, the present study is the first family-based association study between the MTHFR gene and craniosynostosis. The study of single nucleotide polymorphism MTHFR C677T polymorphism in craniosynostosis has not been reported yet. Our results showed that, based on single-marker frequency analysis, C677T polymorphism was not associated with craniosynostosis. In summary, MTHFR is associated with higher plasma homocysteine, a well-known mediator of neuronal damage and brain atrophy. Our results do not support the hypothesis that the MTHFR 677CRT polymorphism plays any role in the development of craniosynostosis. However our results do suggest that most mutations were transferred through the mother to their babies. When taking the results of posterior power calculations into account, they even contradict a causative role of this polymorphism within the bounds of its estimated effect size level. In complex genetic traits, exogenous factors often do play a relevant etiologic role, and if these factors are not prevalent in the population under investigation, the role of interacting co-causative genetic factors might be missed. Our findings revealed that C677T polymorphism of the MTHFR gene was not directly involved in the pathogenesis of craniosynostosis in our Indian population. From the viewpoint of the findings of animal, clinical and pharmacological studies, MTHFR has been hypothesized as a susceptible molecule for craniosynostosis. However, the studies performed until date have produced inconsistent results. To shed light on the potential etiological role of MTHF genetic variants in the pathophysiological mechanism of craniosynostosis, studies in large, homogenous and well-characterized samples are warranted.
 Conclusion Conclusion | |  |
C667T polymorphism of the MTHFR gene is unlikely to play a role in the pathogenesis of craniosynostosis though maternal MTHFR C677T polymorphism may be a genetic risk factor.
 References References | |  |
| 1. | Botto LD, Yang Q. 5,10-Methylenetetrahydrofolate reductase gene variants and congenital anomalies: A HuGE review. Am J Epidemiol 2000;151:862-77.  |
| 2. | Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, et al. A candidate genetic risk factor for vascular disease: A common mutation in methylenetetrahydrofolate reductase. Nat Genet 1995;10:111-3.  [ PUBMED] |
| 3. | Frosst P, Zhang Z, Pai A, Rozen R. The methylenetetrahydrofolate reductase (Mthfr) gene maps to distal mouse chromosome 4. Mamm Genome 1996;7:864-5.  |
| 4. | Cohen MM. Craniosynostosis: Diagnosis, Evaluation, and Management. New York: Oxford University Press;2000.  |
| 5. | Johnson D, Wall SA, Mann S, Wilkie AO. A novel mutation, Ala315Ser, in FGFR2: A gene-environment interaction leading to craniosynostosis? Eur J Hum Genet 2000;8:571-7.  |
| 6. | Seto ML, Hing AV, Chang J, Hu M, Kapp-Simon KA, Patel PK, et al. Isolated sagittal and coronal craniosynostosis associated with TWIST box mutations. Am J Med Genet A 2007;143:678-86.  |
| 7. | Weber I, Ninkovic M, Janicke A, Utermann B, Witsch Baumgartner M, Anderl H, et al. Molecular analysis of 74 patients with craniosynostosis. Eur J Hum Genet 2001;9 Suppl 1:P0409, 179.  |
| 8. | Merrill AE, Bochukova EG, Brugger SM, Ishii M, Pilz DT, Wall SA, et al. Cell mixing at a neural crest-mesoderm boundary and deficient ephrin-Eph signaling in the pathogenesis of craniosynostosis. Hum Mol Genet 2006;15:1319-28.  |
| 9. | Wilkie AO, Bochukova EG, Hansen RM, Taylor IB, Rannan-Eliya SV, Byren JC, et al. Clinical dividends from the molecular genetic diagnosis of craniosynostosis. Am J Med Genet A 2007;143A:1941-9.  |
| 10. | Mefford HC, Shafer N, Antonacci F, Tsai JM, Park SS, Hing AV, et al. Copy number variation analysis in single-suture craniosynostosis: Multiple rare variants including RUNX2 duplication in two cousins with metopic craniosynostosis. Am J Med Genet A 2010;152A:2203-10.  |
| 11. | Vissers LE, Cox TC, Maga AM, Short KM, Wiradjaja F, Janssen IM, et al. Heterozygous mutations of FREM1 are associated with an increased risk of isolated metopic craniosynostosis in humans and mice. PLoS Genet 2011;7:e1002278.  |
| 12. | Kim SD, Liu JL, Roscioli T, Buckley MF, Yagnik G, Boyadjiev SA, et al. Leucine-rich repeat, immunoglobulin-like and transmembrane domain 3 (LRIT3) is a modulator of FGFR1. FEBS Lett 2012;586:1516-21.  |
| 13. | Melville H, Wang Y, Taub PJ, Jabs EW. Genetic basis of potential therapeutic strategies for craniosynostosis. Am J Med Genet A 2010;152A:3007-15.  |
| 14. | Wilkie AO, Byren JC, Hurst JA, Jayamohan J, Johnson D, Knight SJ, et al. Prevalence and complications of single-gene and chromosomal disorders in craniosynostosis. Pediatrics 2010;126:e391-400.  |
| 15. | Passos-Bueno MR, Serti Eacute AE, Jehee FS, Fanganiello R, Yeh E. Genetics of craniosynostosis: Genes, syndromes, mutations and genotype-phenotype correlations. Front Oral Biol 2008;12:107-43.  |
| 16. | Rajagopalan P, Jahanshad N, Stein JL, Hua X, Madsen SK, Kohannim O, et al. Common folate gene variant, MTHFR C677T, is associated with brain structure in two independent cohorts of people with mild cognitive impairment. Neuroimage Clin 2012 4;1:179-87.  |
| 17. | Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning a Laboratory Manual. 2 nd ed. New York: Cold Spring Harbor Laboratory; 1989.  |
| 18. | Thangaraj K, Joshi MB, Reddy AG, Gupta NJ, Chakravarty B, Singh L. CAG repeat expansion in the androgen receptor gene is not associated with male infertility in Indian populations. J Androl 2002;23:815-8.  |
| 19. | Reutter H, Birnbaum S, Lacava AD, Mende M, Henschke H, Bergé S, et al. Family-based association study of the MTHFR polymorphism C677T in patients with nonsyndromic cleft lip and palate from central Europe. Cleft Palate Craniofac J 2008;45:267-71.  |
| 20. | Reutter H, Becker T, Ludwig M, Schäfer N, Detlefsen B, Beaudoin S, et al. Family-based association study of the MTHFR polymorphism C677T in the bladder-exstrophy-epispadias-complex. Am J Med Genet A 2006;140:2506-9.  |
[Figure 1], [Figure 2]
[Table 1]
|






