| |


 |
| Year : 2014 | Volume
: 8
| Issue : 4 | Page : 127-132 |
|
|
|
|
|
SURGICAL TECHNIQUE Iliac crest allograft glenoid reconstruction for recurrent anterior shoulder instability in athletes: Surgical technique and results
Randy Mascarenhas1, Eden Raleigh2, Sheila McRae3, Jeff Leiter3, Bryan Saltzman4
1 University of Texas Health Sciences Center at Houston, Houston, Australia
2 City Orthopaedics, Melbourne, Australia
3 Pan Am Clinic, Winnipeg, Manitoba, Canada
4 Rush University Medical Center, Chicago, IL, Australia
Correspondence Address:
Randy Mascarenhas
5656 Kelley Street, Houston, TX, 77019
Australia
 Source of Support: None, Conflict of Interest: None  | 2 |
DOI: 10.4103/0973-6042.145269

|
|
|
|
| Date of Web Publication | 21-Nov-2014 |
 Abstract Abstract | | |
Performing a labral repair alone in patients with recurrent anterior instability and a large glenoid defect has led to poor outcomes. We present a technique involving the use of the iliac crest allograft inserted into the glenoid defect in athletes with recurrent anterior shoulder instability and large bony defects of the glenoid (>25% of glenoid diameter). All athletes with recurrent anterior shoulder instability and a large glenoid defect that underwent open anterior shoulder stabilization and glenoid reconstruction with the iliac crest allograft were followed over a 4-year period. Preoperatively, a detailed history and physical exam were obtained along with standard radiographs and magnetic resonance imaging of the affected shoulder. All patients also completed the Simple Shoulder Test (SST) and American Shoulder and Elbow Surgeons (ASES) evaluation forms preoperatively. A computed tomography scan was obtained postoperatively to assess osseous union of the graft and the patient again went through a physical exam in addition to completing the SST, ASES, and Western Ontario Shoulder Instability Index (WOSI) forms. 10 patients (9 males, 1 female) were followed for an average of 16 months (4-36 months) and had a mean age of 24.4 years. All patients exhibited a negative apprehension/relocation test and full shoulder strength at final follow-up. Eight of 10 patients had achieved osseous union at 6 months (80.0%). ASES scores improved from 64.3 to 97.8, and SST scores improved from 66.7 to 100. Average postoperative WOSI scores were 93.8%. The use of the iliac crest allograft provides a safe and clinically useful alternative compared to previously described procedures for recurrent shoulder instability in the face of glenoid deficiency.
Keywords: Glenoid bone loss, the iliac crest allograft, shoulder instability
How to cite this article:
Mascarenhas R, Raleigh E, McRae S, Leiter J, Saltzman B. Iliac crest allograft glenoid reconstruction for recurrent anterior shoulder instability in athletes: Surgical technique and results. Int J Shoulder Surg 2014;8:127-32 |
How to cite this URL:
Mascarenhas R, Raleigh E, McRae S, Leiter J, Saltzman B. Iliac crest allograft glenoid reconstruction for recurrent anterior shoulder instability in athletes: Surgical technique and results. Int J Shoulder Surg [serial online] 2014 [cited 2016 Aug 23];8:127-32. Available from: http://www.internationalshoulderjournal.org/text.asp?2014/8/4/127/145269 |
 Introduction Introduction | |  |
Current treatment of uncomplicated Bankart lesions involves repair to the glenoid with or without a capsular shift and is generally very successful, with recurrence rates published at between 5% and 10%. [1] There is, however, a group of patients who respond poorly to simple Bankart repair. Failure rates of labral repair alone in the setting of large glenoid defects have been reported to be up to 67%. [1]
Techniques such as the Bristow, Latarjet, Trillat, and various bone grafting procedures related to operative treatment of anterior shoulder instability with significant glenoid bone loss have been documented. [2],[3],[4],[5],[6],[7],[8] Bigliani et al. performed coracoid transfer for glenoid bone defects involving more than 25% of the glenoid surface. [9] Similarly, Itoi et al. [10],[11] recommended bone grafting in the setting of shoulder instability and a significant glenoid defect after observing that osseous defects wider than 21% of the glenoid length caused shoulder instability and limited range of motion after Bankart repair alone. However, arthritis, hardware failure, range of motion deficits, and nonunion have been associated with procedures that involve the transfer of the coracoid process. [12] Anatomic glenoid reconstruction may be difficult with these nonanatomical approaches as well and may complicate further surgery if needed.
Our technique involves using the iliac crest allograft inserted into the glenoid defect in large bony defects (>25% of glenoid diameter), helping to contain the humerus in the glenoid. This method removes donor-site morbidity of autogenous reconstructions and complications associated with coracoid transfer procedures, which provide nonanatomic solutions and have been associated with loss of shoulder motion and the development of arthritis. [12] We hypothesized that restoring a near-normal glenoid contour with iliac crest allograft would prevent further dislocations with a high rate of osseous union and lead to positive outcomes with respect to shoulder range of motion, strength, and patient self-reported outcome measures.
 Surgical technique Surgical technique | |  |
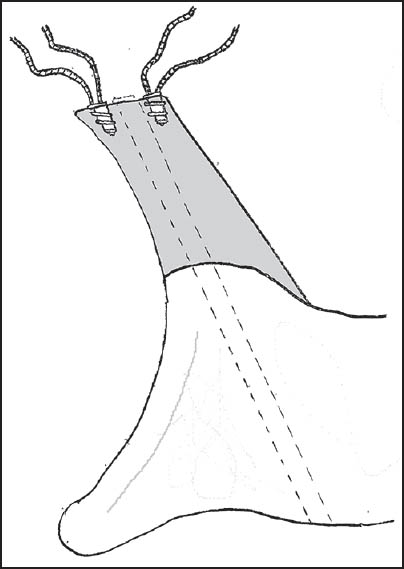
Patients were seated in the beach-chair position. A scalene block and general anaesthetic were used in all patients. An examination under anesthesia of the shoulder was performed to confirm the positions of instability and the presence of contralateral instability. Preoperative cefazolin was administered, and the involved arm was free-draped and prepped with betadine. A standard deltopectoral incision was used, and subscapularis reflected with stay sutures attached for later repair [Figure 1]. The capsular layer was separated, and a vertical capsulotomy was used to gain access to the glenoid. A Fukuda retractor was used to retract the humeral head, and an assessment was made of the glenohumeral articulation [Figure 2]. The presence of a Hills-Sachs lesion was documented, and the humerus externally rotated to assess whether this lesion engaged the glenoid. A measurement of the glenoid bone loss was made with a depth-gauge, using the bare area as the true center of the inferior glenoid. Any previous loose hardware was removed, and the glenoid surface prepared with a periosteal elevator. The allograft bone was refashioned with a small burr to allow conformity and ensure a congruent articular surface [Figure 3]. Anterior-posterior (A-P) glenoid bone loss was corrected, and the concavity of the iliac crest provided a very close approximation of the normal glenoid concavity. Two Bio Mini-Revo (Conmed Linvatech, Florida) suture anchors were then inserted into the glenoid at either ends of the base of the allograft [Figure 4]. These would be used later to repair the labrum. The shaped allograft was predrilled using a 2.5 mm drill bit and fixed to the glenoid using two 4.0 mm partially threaded cancellous screws with washers each penetrating two cortices [Figure 5]. The suture anchors were then used to repair the labrum onto the graft to create an intra-articular graft [Figure 6]. Standard closure was performed with nonabsorbable repair of the subscapularis followed by absorbable suture closure of the approach. 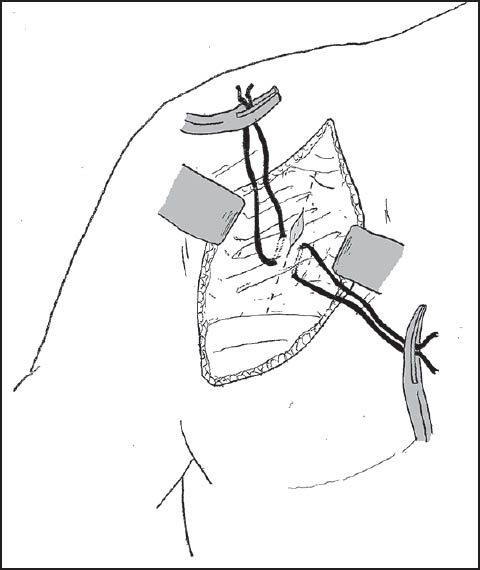 | Figure 1: A standard deltopectoral incision was utilized with stay sutures attached to the subscapularis as it is incised and retracted to allow for later repair
Click here to view |
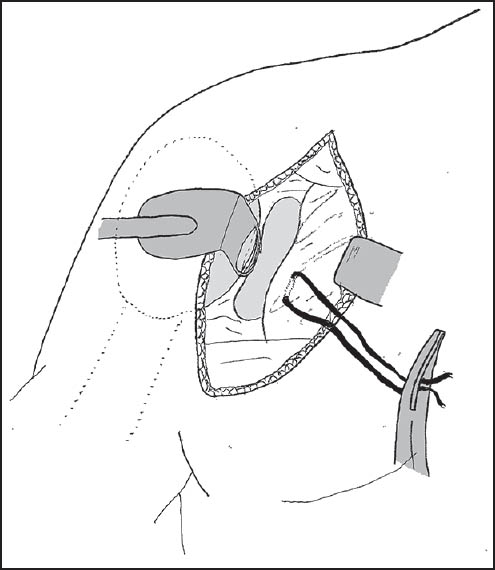 | Figure 2: A Fukuda retractor is used to retract the humeral head to allow assessment of the glenohumeral articulation and measurement of glenoid bone loss
Click here to view |
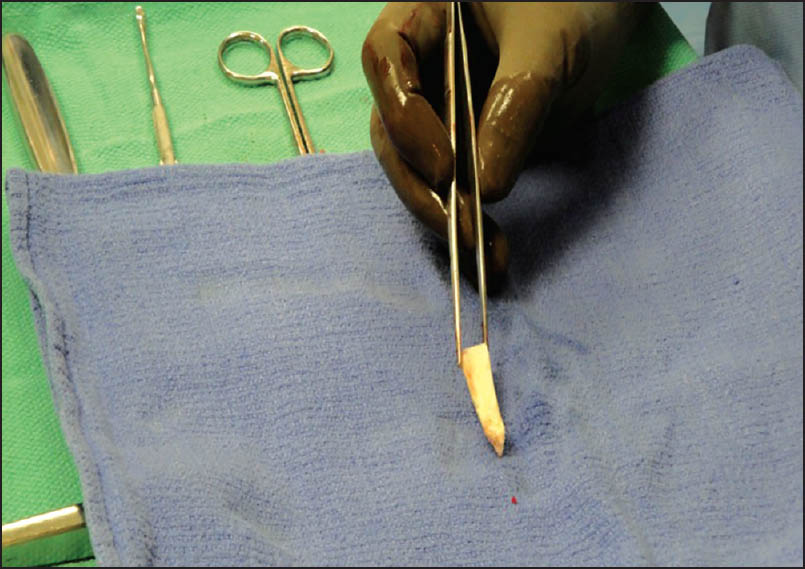 | Figure 3: The allograft bone is refashioned with a small burr to allow conformity and ensure a congruent articular surface
Click here to view |
 | Figure 4: Two suture anchors were inserted into the glenoid (seen here in an axial view) at either ends of the allograft base to allow subsequent repair of the labrum
Click here to view |
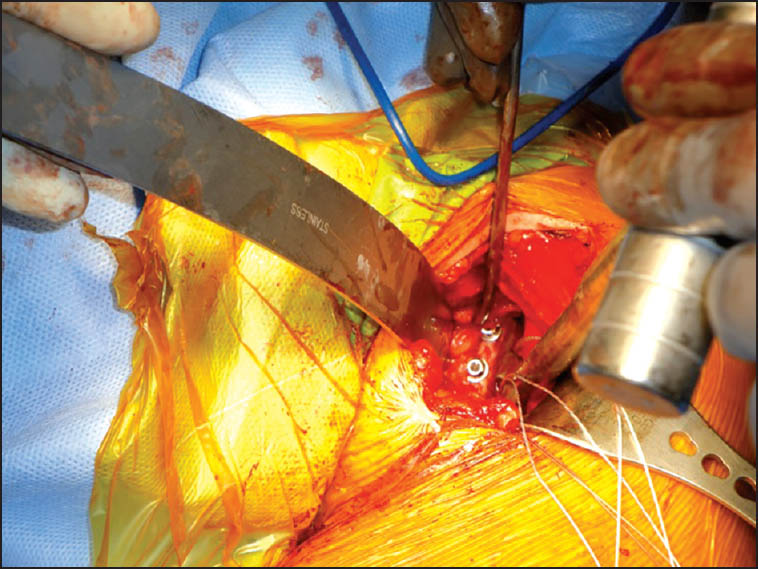 | Figure 5: The shaped allograft is predrilled using a 2.5 mm drill bit and fixed to the glenoid using two 4.0 mm partially threaded cancellous screws with washers, each penetrating two cortices
Click here to view |
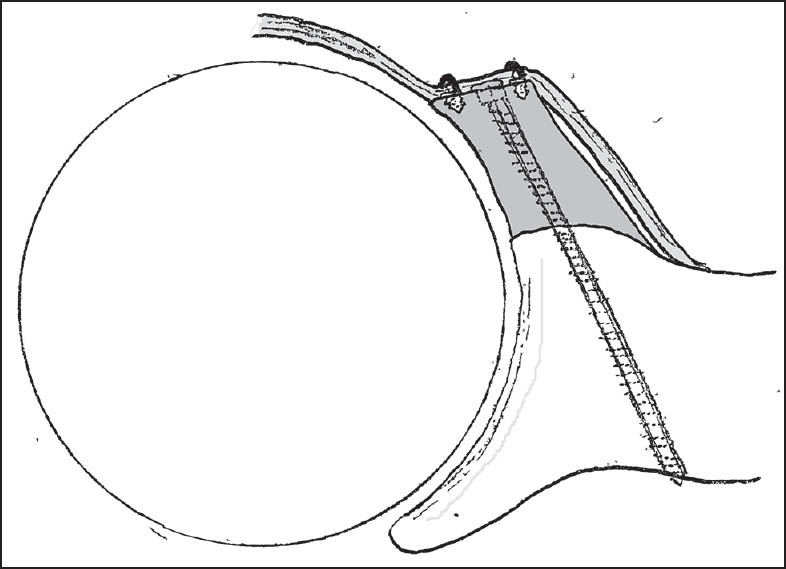 | Figure 6: The suture anchors were used to create an intra-articular graft with repair of the labrum over the graft (seen here in an axial view)
Click here to view |
Postoperative care
Patients were given a four-stage exercise program [Table 1]. A sling was used for 4 weeks with only pendulum exercises for the first 2 weeks. Gradual supervised physiotherapy involved active and passive-assisted range of motion exercises. Restrictions on strength training remained for at least 4 months, and return to sports was not allowed for 6 months and until computed tomography (CT) scan showed osseous union of the allograft. All patients received wound checks at 10 days, and follow-up appointments at 6 weeks, 3 months, 6 months, and yearly after the surgery.
Potential complications
The procedure carries with it the standard surgical risks of superficial and deep wound infection. The axillary nerve is at risk when preparing the anteroinferior glenoid defect. Patients should be counseled about postoperative loss of external rotation, and stiffness can occur if appropriate rehabilitation is not performed. The subscapularis repair must be protected during the first four postoperative weeks and then gradual range of motion exercises should begin. If bicortical graft fixation is not achieved, there exists a risk of graft migration over time. In addition, graft resorption and hardware failure/migration can also occur. Finally, surgeons should take care to ensure that that the graft is congruent with the native glenoid and that there are no prominences that could lead to arthrosis over time.
 Results Results | |  |
Evaluation
Ten consecutive patients with recurrent anterior shoulder instability who underwent surgery at our institution between 2005 and 2009 were evaluated. Suspicion of glenoid bone loss was based on the high number of recurrences, decreasing the force required for instability, and prior failed arthroscopic procedures. All patients had positive apprehension and relocation [13],[14] signs. In addition, all patients had normal rotator cuff function, and no patient had associated rotator cuff injury or neurovascular deficit.
All patients had significant anterior glenoid bone loss, and all initial injuries occurred at least 6 months before surgery. All patients gave informed consent, and all procedures were performed by the same senior surgeon at the same institution. Mean age was 24.4 years (range: 17-37) with six dominant shoulders affected. Five of the 10 patients had undergone prior arthroscopic stabilizations that had failed. Diagnoses were made by means of history, physical examination, and imaging studies. All patients sustained their instability as a result of trauma sustained during a sporting event [Table 2].
In addition to preoperative plain radiographs (including true A-P glenoid views, axillary views, and Stryker-Notch views), all patients had magnetic resonance imaging (MRI) performed. Preoperative MRI and intra-operative depth-gauge measurements were used to assess the true degree of glenoid deficiency. All patient MRI results were documented by the senior author. The oblique-sagittal and axial slices were used, and anterior bone loss extrapolated by drawing a circle based on the bare-area. The distance of the remaining A-P diameter of the circle was compared to the circle diameter to provide a percentage. This helped in the calculation of glenoid bone loss in the A-P plane. Intra-operatively, a depth-gauge was used to calculate the true bone loss by comparing the A-P diameter at the bare area and comparing that to twice the radius of the distance from the bare area to posterior glenoid in the same plane.
Patients were evaluated pre- and postoperatively using the American Shoulder and Elbow Surgeons (ASES) self-assessment score [15] and Simple Shoulder Test (SST). [13] The Western Ontario Shoulder Instability Index (WOSI) [16] was also administered postoperatively. In addition, patients were evaluated postoperatively by one observer. Measurement of range of motion was performed with the patient supine for internal and external rotation and with the patient standing for flexion. Strength was measured by manual testing and an anterior apprehension, and relocation test [13] was performed. True A-P glenoid and axillary views were obtained immediately after, 6 weeks after, and 3 months after surgery to evaluate the fixation of screws and suture anchors and follow the progression of bone healing. At 4-6 months after surgery, all patients underwent a CT scan to confirm osseous union, concentric glenohumeral articulation, and hardware position.
Clinical outcomes
Information on ten patients was reviewed. Nine patients were male and one female with an average age of 24.4 years. Etiology of injury for all patients was traumatic and sports related. Average postoperative follow-up was 16 months postsurgery (range: 4-36 months). Five of 10 patients had previous arthroscopic shoulder stabilizations that had failed. Preoperative ASES and SST scores were 64.3% (range: 46.7-100) and 66.7% (range: 25-91.7), respectively [Table 2]. Postoperative ASES and SST scores were 97.8% (range: 96.7-100) and 100%, respectively [Table 3]. Average WOSI score was 93.8% (92.1-95.5). Mean loss of external rotation in abduction was 16.3° (range: 0-55). Strength was assessed postoperatively with five subjects exhibiting grade 4 strength and five subjects exhibiting grade 5 strength. In those individuals with grade 4 strength, internal rotation and forward flexion strength were slightly diminished. None of the patients experienced further shoulder dislocation or feelings of postoperative apprehension. All patients returned to their preinjury level of athletic activity.
Follow-up radiographic analysis
Postoperative plain radiographs and CT scans at 4-6 months postsurgery confirmed that the graft appeared to incorporate along the anterior glenoid rim and restored glenoid contour in all but two patients. Both of these patients were asymptomatic and did not require operative intervention. In addition, there was no evidence of joint space narrowing, no articular impingement by the screws in any of the patients, and no evidence of screw breakage. Two patients had small degrees of osteolysis around the screws that eventually required screw removal due to shoulder pain. Symptoms resolved after removal, and the graft was noted to have incorporated intraoperatively at the time of screw removal in both patients.
 Discussion Discussion | |  |
The glenoid cavity plays two roles in glenohumeral motion. Its deepening effect resists shear forces to avoid anterior dislocation and glenoid arc length allows the glenoid cavity to resist axial forces at various humeral angles. [1] Several biomechanical studies have established the importance of the anteroinferior glenoid in glenohumeral stability, and the associated need for anatomic glenoid reconstruction in cases with large anterior glenoid defects. [17],[18],[19],[20] Bigliani et al. [9] recommended coracoid transfer for rim defect lesions that exceeded 25% of the glenoid surface. Itoi et al. [11] showed that osseous defects wider than 21% of glenoid length may cause instability and limit shoulder motion after simple capsular repair. Hovelius et al. measured the size of anteroinferior bony Bankart lesions by CT and emphasized the need for bone grafting. [12] Burkhart and De Beer identified significant glenoid osseous defects as having an inverted-pear appearance and concluded that the risk of failure of arthroscopic procedures alone in this patient population was high. [1] In an anatomic study including patients and cadavers, Lo et al. reported that the inverted-pear glenoid appearance represents significant bone loss of at least 25-27% of the inferior glenoid width. [21]
Quantifying the amount of glenoid bone loss has historically been done using both imaging and arthroscopy. Burkhart et al. [1],[21],[22] have established methods of assessing the amount of bone loss required to produce an inverted pear glenoid. The normal glenoid shape is similar to a pear, with a lower A-P diameter larger than the upper A-P diameter. When the anterior glenoid bone loss reaches 28.8 ± 1.1%, the shape changes so that the upper A-P diameter is larger; hence the inverted pear-shape. [21],[22] This change has been reported to result in an increase in the failure rate of arthroscopic Bankart repair from 4% to 61%. [1] More recently, Gerber and Nyffeler [17] presented a new method for assessment of glenoid bone loss and determined its importance to glenohumeral joint stability.
Both the Bristow and the Latarjet procedures involve transfer of the coracoid process through the subscapularis tendon and onto the anterior scapular neck. These coracoid process transfers restore stability through the use of an anterior bone block coupled with the passive sling effect and active stabilizing effect provided by the conjoined tendon. Several series have reported very satisfactory outcomes with these techniques, but others have observed significant problems including recurrent instability, loss of motion, impingement, hardware failure, nonunion, and arthritis. [2],[3],[4],[5],[6],[7],[8],[12]
Warner et al. [23] described a series of 11 patients who underwent anatomic reconstruction of the glenoid with autogenous iliac crest bone graft for recurrent shoulder instability. Overall, no patients reported recurrent instability and CT demonstrated union of the bone graft with incorporation along the anterior glenoid rim and preservation of joint space in all patients. Similarly, Weng et al. reported on nine patients at 4.5-14 years follow-up who underwent glenoid reconstruction with femoral head allograft for recurrent shoulder instability. All grafts achieved union within 6 months of surgery, and two patients had further dislocations. [24]
Our technique of anatomic reconstruction of the deficient anterior glenoid using the iliac crest allograft was developed in response to some of the limitations seen with the coracoid process transfer procedures and has been successful in our experience (See [Table 4] for surgical pearls). While long-term effects of the procedure are yet to be seen, current results seem to suggest that the graft seems to lead to a high rate of successful union and stability. In the present study, the subjective outcome was satisfactory in all patients and all were able to return to sports participation. No recurrent instability was observed, and no evidence of hardware failure or joint degeneration was noted from radiography. In addition, allograft bone avoids the complications associated with donor site harvest and may be useful in situations with anterior glenoid defects involving more than one-third of the articular surface. Edwards and Walch have suggested that glenoid defects involving more than one-third of the articular surface should undergo a reconstructive procedure rather than a coracoid transfer. [25]
There are some limitations to our results. We did not have preoperative WOSI scores and had a small sample size and lack of a comparison group. In addition, two patients required screw removal secondary to symptomatic hardware, but both were still noted to have a stable fibrous union at the time of hardware removal. Although a high union rate and good stability for allogeneic bone grafting have shown good short-term outcomes in our study, long-term effects are yet unknown. The long-term outcomes could include degenerative disease of the glenohumeral joint, resulting from possible graft impingement on the humeral head, and this phenomenon will have to be evaluated in long-term follow-up.
 Conclusion Conclusion | |  |
We conclude that this surgical technique utilizing the iliac crest allograft for addressing anterior glenoid bone loss is a reasonable alternative to coracoid transfer procedures for treatment of shoulder instability in the setting of anterior glenoid insufficiency.
 References References | |  |
| 1. | Burkhart SS, De Beer JF. Traumatic glenohumeral bone defects and their relationship to failure of arthroscopic Bankart repairs: Significance of the inverted-pear glenoid and the humeral engaging Hill-Sachs lesion. Arthroscopy 2000;16:677-94.  |
| 2. | Allain J, Goutallier D, Glorion C. Long-term results of the Latarjet procedure for the treatment of anterior instability of the shoulder. J Bone Joint Surg Am 1998;80:841-52.  |
| 3. | Gerber C, Terrier F, Ganz R. The Trillat procedure for recurrent anterior instability of the shoulder. J Bone Joint Surg Br 1988;70:130-4.  |
| 4. | Hovelius LK, Sandström BC, Rösmark DL, Saebö M, Sundgren KH, Malmqvist BG. Long-term results with the Bankart and Bristow-Latarjet procedures: recurrent shoulder instability and arthropathy. J Shoulder Elbow Surg 2001;10:445-52.  |
| 5. | Hovelius L, Sandström B, Sundgren K, Saebö M. One hundred eighteen Bristow-Latarjet repairs for recurrent anterior dislocation of the shoulder prospectively followed for fifteen years: Study I - Clinical results. J Shoulder Elbow Surg 2004;13:509-16.  |
| 6. | Nielson AB, Nielsen K. The modified Bristow procedure for recurrent anterior dislocation of the shoulder. Results and complications. Acta Orthop Scand 1982;53:229-32.  |
| 7. | Schauder KS, Tullos HS. Role of the coracoid bone block in the modified Bristow procedure. Am J Sports Med 1992;20:31-4.  |
| 8. | Singer GC, Kirkland PM, Emery RJ. Coracoid transposition for recurrent anterior instability of the shoulder. A 20-year follow-up study. J Bone Joint Surg Br 1995;77:73-6.  |
| 9. | Bigliani LU, Newton PM, Steinmann SP, Connor PM, Mcllveen SJ. Glenoid rim lesions associated with recurrent anterior dislocation of the shoulder. Am J Sports Med 1998;26:41-5.  |
| 10. | Itoi E, Lee SB, Amrami KK, Wenger DE, An KN. Quantitative assessment of classic anteroinferior bony Bankart lesions by radiography and computed tomography. Am J Sports Med 2003;31:112-8.  |
| 11. | Itoi E, Lee SB, Berglund LJ, Berge LL, An KN. The effect of a glenoid defect on anteroinferior stability of the shoulder after Bankart repair: A cadaveric study. J Bone Joint Surg Am 2000;82:35-46.  |
| 12. | Young DC, Rockwood CA Jr. Complications of a failed Bristow procedure and their management. J Bone Joint Surg Am 1991;73:969-81.  |
| 13. | Jobe FW, Bradley JP. The diagnosis and nonoperative treatment of shoulder injuries in athletes. Clin Sports Med 1989;8:419-38.  |
| 14. | Speer KP, Hannafin JA, Altchek DW, Warren RF. An evaluation of the shoulder relocation test. Am J Sports Med 1994;22:177-83.  |
| 15. | Richards RR, An KN, Bigliani LU, Friedman RJ, Gartsman GM, Gristina AG, et al. A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg 1994;3:347-52.  |
| 16. | Kirkley A, Griffin S, McLintock H, Ng L. The development and evaluation of a disease-specific quality of life measurement tool for shoulder instability. The Western Ontario Shoulder Instability Index (WOSI). Am J Sports Med 1998;26:764-72.  |
| 17. | Gerber C, Nyffeler RW. Classification of glenohumeral joint instability. Clin Orthop Relat Res 2002; 65-76.  |
| 18. | Lazarus MD, Sidles JA, Harryman DT 2 nd , Matsen FA 3 rd . Effect of a chondral-labral defect on glenoid concavity and glenohumeral stability. A cadaveric model. J Bone Joint Surg Am 1996;78:94-102.  |
| 19. | Lippitt SB, Vanderhooft JE, Harris SL, Sidles JA, Harryman DT 2 nd , Matsen FA 3 rd . Glenohumeral stability from concavity-compression: A quantitative analysis. J Shoulder Elbow Surg 1993;2:27-35.  |
| 20. | Lusardi DA, Wirth MA, Wurtz D, Rockwood CA Jr. Loss of external rotation following anterior capsulorrhaphy of the shoulder. J Bone Joint Surg Am 1993;75:1185-92.  |
| 21. | Lo IK, Parten PM, Burkhart SS. The inverted pear glenoid: An indicator of significant glenoid bone loss. Arthroscopy 2004;20:169-74.  |
| 22. | Burkhart SS, Debeer JF, Tehrany AM, Parten PM. Quantifying glenoid bone loss arthroscopically in shoulder instability. Arthroscopy 2002;18:488-91.  |
| 23. | Warner JJ, Gill TJ, O′hollerhan JD, Pathare N, Millett PJ. Anatomical glenoid reconstruction for recurrent anterior glenohumeral instability with glenoid deficiency using an autogenous tricortical iliac crest bone graft. Am J Sports Med 2006;34:205-12.  |
| 24. | Weng PW, Shen HC, Lee HH, Wu SS, Lee CH. Open reconstruction of large bony glenoid erosion with allogeneic bone graft for recurrent anterior shoulder dislocation. Am J Sports Med 2009;37:1792-7.  |
| 25. | Edwards TB, Walch G. The Latarjet procedure for recurrent anterior shoulder instability: Rationale and technique. Oper Tech Sports Med 2002;10:25-32.  |
[Figure 1], [Figure 2], [Figure 3], [Figure 4], [Figure 5], [Figure 6]
[Table 1], [Table 2], [Table 3], [Table 4]
|
