| |


 |
| Year : 2015 | Volume
: 9
| Issue : 4 | Page : 135-138 |
|
|
|
|
|
CASE REPORT Giant cell tumor of the humeral head treated by denosumab: Implication to shoulder surgeons
Ka Hei Leung1, Albert Ying Lee Lam1, Kenneth Wai Yip Ho1, Tony Wai Hung Shek2
1 Department of Orthopaedics and Traumatology, Division of Anatomical Pathology, Queen Mary Hospital, Hong Kong
2 Department of Pathology, Division of Anatomical Pathology, Queen Mary Hospital, Hong Kong
Correspondence Address:
Ka Hei Leung
Department of Orthopaedics and Traumatology, Queen Mary Hospital, 102 Pokfulam Road
Hong Kong
 Source of Support: None, Conflict of Interest: None
DOI: 10.4103/0973-6042.167956
 |
|
|
|
| Date of Web Publication | 22-Oct-2015 |
 Abstract Abstract | | |
Giant cell tumor is a benign bone tumor that is commonly encountered. The optimal treatment of a giant cell tumor which causes extensive bony destruction is controversial. Recent studies on the receptor activator of nuclear factor κB ligand antagonist denosumab may offer a new treatment option for these patients. We presented a patient with giant cell tumor of the humeral head. He was initially treated with denosumab and subsequently with the operation. The shoulder joint was successfully salvaged. But there are potential difficulties that surgeons may face in patients treated with denosumab.
Keywords: Denosumab, extended curettage, giant cell tumor, joint preservation surgery, receptor activator of nuclear factor κB ligand antagonist
How to cite this article:
Leung KH, Lam AY, Ho KW, Shek TW. Giant cell tumor of the humeral head treated by denosumab: Implication to shoulder surgeons. Int J Shoulder Surg 2015;9:135-8 |
 Introduction Introduction | |  |
Giant cell tumor (GCT) accounts for 5-8% of all primary bone tumors. [1] It usually occurs between 20 and 40 years of age and is commonly located at the distal femur, proximal tibia or distal radius. Proximal humerus is a rare site of involvement, accounting for only around 4% of the disease. [2] GCT presents as a lytic and eccentric lesion at the epiphyseal region of long bones. The lesion is often expansile and causes thinning of the cortex. Pain and pathological fractures are the usual presentations. Histologically, GCTs are composed of two distinct cell types: Mononuclear stromal cells and osteoclast-like multinucleated giant cells. [3],[4] The stromal cells are thought to be the principal tumor cells. They express receptor activator of nuclear factor κB (RANK) ligand and recruit monocytes to the site of the tumor. RANK receptors are present on the surface of monocytes. RANK ligands bind to RANK receptors and induce the differentiation of monocytes into multinucleated giant cells. The giant cells have properties of osteoclasts. They mediate the bone resorption and are responsible for the purely lytic feature seen on radiographs.
Denosumab is a RANK ligand antagonist. It is a fully human, synthetic IgG antibody that binds to RANK ligand with high affinity, preventing its interaction with RANK receptor. [5],[6] The first large-scale study of its use was in osteoporotic patients. [7] In that study, denosumab was shown to increase the bone density. Rare but major adverse effects include osteonecrosis of the jaw and hypocalcemia, which occur in around 2% of patients receiving denosumab. [8] Denosumab had been evaluated in a small scale study in patients with GCT of bone. [9] In that study, Thomas et al. showed that 30 of 35 patients had a response which was defined as more than 90% elimination of giant cells or an absence of radiological progression. However, there is a little study in the orthopedic literature regarding how this is relevant to our practice. We present here a patient with GCT of the humeral head. He was treated with denosumab and subsequently underwent an extended curettage of the lesion. The patient agreed for publication of his case material.
 Case Report Case Report | |  |
A 62-year-old man complained of spontaneous onset left shoulder pain and stiffness for around 2 weeks. X-ray showed an expansile and lytic lesion over the proximal humerus [Figure 1]. The cortical bone was so thin that it could not be well delineated. Magnetic resonance imaging (MRI) showed a T2 hyperintense expansile mass. The humeral head was deformed, but the lesion remained intraosseous. There was no extraosseous soft tissue component. Open biopsy of the lesion confirmed GCT [Figure 2].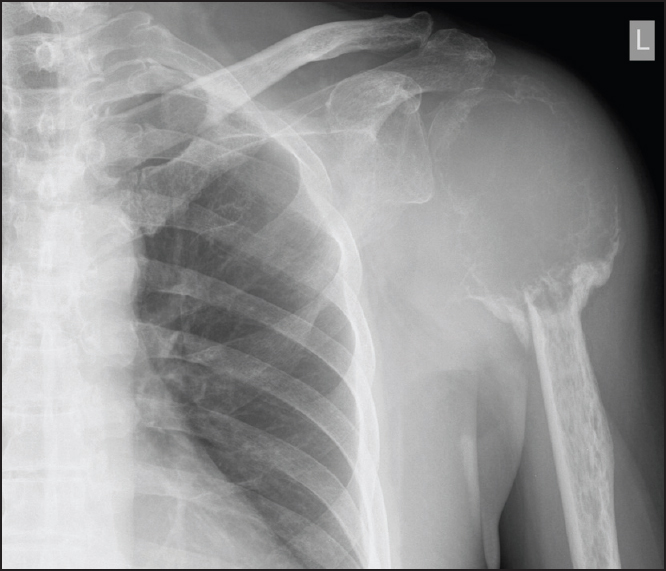 | Figure 1: Expansile and lytic lesion over the left shoulder with cortex poorly delineated
Click here to view |
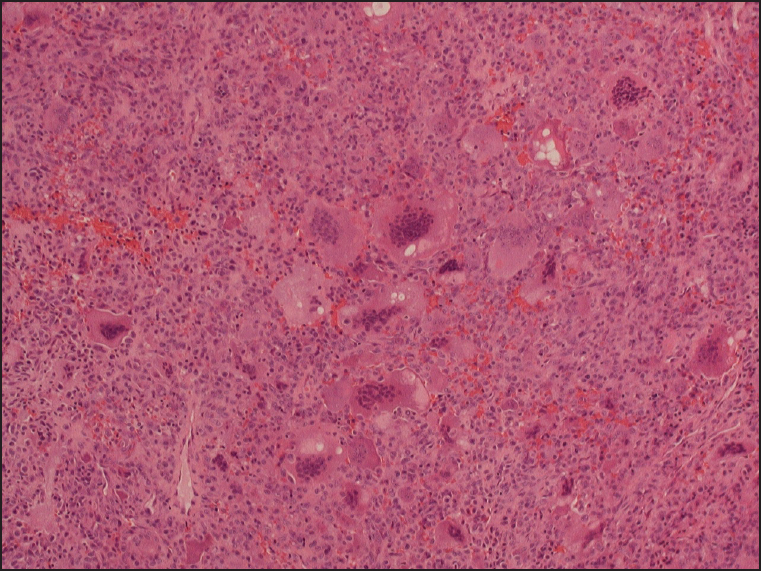 | Figure 2: Giant cell tumor showing the multinucleated giant cells in a background of mononuclear cells (H and E, ×40)
Click here to view |
Treatment options of extended curettage versus wide local excision were discussed with the patient. He opted for joint preservation procedures. The patient also requested to try denosumab as a neoadjuvant treatment after we presented to him a few recent reports which showed that denosumab could induce ossification in GCT. Informed consent was obtained, and the patient received 6 doses of denosumab (120 mg subcutaneous injection on day 0, day 6, day 13, day 29, day 57, and day 85, respectively). The dosage and interval were similar to the protocol used by Thomas et al. [9] Clinically, the left shoulder pain decreased although the motion was still limited. He was put on calcium and multivitamin supplement, and regular blood tests showed normal renal function and calcium level. The patient did not experience any side effect. Regular X-ray showed no progression of the lesion. In addition, the lesion became more sclerotic. There was progressive bone deposition in the tumor, and the cortex became more clearly delineated [Figure 3]a-c. A reassessment MRI showed no interval change in size or signal of the lesion.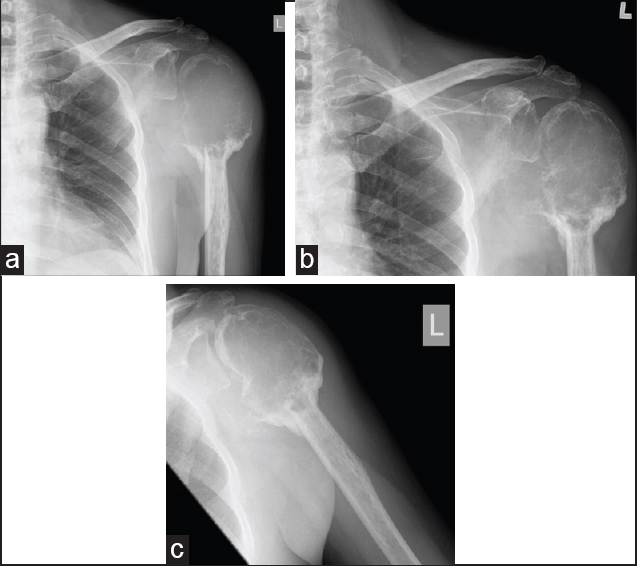 | Figure 3: (a) X-ray at 1-month after initiation of denosumab. (b) X-ray at 3 months after initiation of denosumab. (c) X-ray at 4 months after initiation of denosumab
Click here to view |
The patient received operation on day 118 (around 4 months) after starting denosumab. An extended curettage was performed through a deltopectoral approach. We opened a cortical window at the metaphyseal area. The tumor tissue inside was found to be extremely hard and was significantly different from the soft texture of the usual GCT. Normally for GCT, we could easily scoop out the tumor tissue by curette. But it was not possible in this case. Rongeur and scalpel were needed to remove most of the tumor tissue until the subchondral and metaphyseal bone was reached. But the difference in texture between the tumor tissue and normal bone was not distinct. High-speed burr was used to lightly debride the surrounding bony cortex. Hydrogen peroxide (H 2 O 2 ) and water (H 2 O) were used for irrigation. Cement and three intra-medullary titanium elastic rods were used to fill up the cavity and also as prophylaxis of fracture. Postoperative X-ray showed no residual lytic lesion. Histological examination of the tumor tissue revealed mainly fibro-osseous tissue. There were interconnected broad trabeculae of bone embedding a small number of stromal cells [Figure 4]. No giant cells were present in the sample.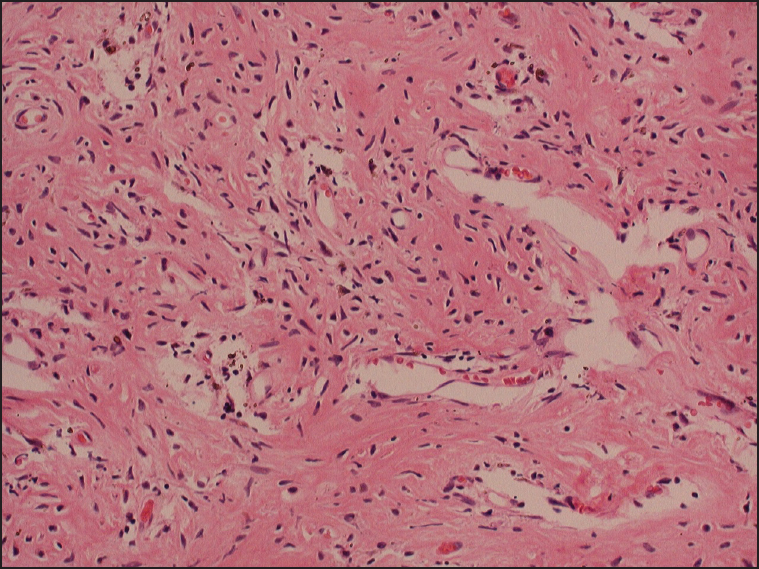 | Figure 4: Posttreatment specimen showing bony matrix with small spindle cells (H and E, ×100)
Click here to view |
The patient received two more doses of denosumab (120 mg) after the operation at 1 and 2 months respectively. Upon follow-up at 2 years postoperation, the patient reported complete resolution of bone pain. The shoulder could flex up to 70° and abduct up to 90°. The patient was satisfied with the functional outcome. There was no recurrence on X-ray [Figure 5] and MRI.
 Discussion Discussion | |  |
Extended curettage is currently the treatment of choice for GCTs. [2],[10] But the local recurrence rate is still around 15-27% even with the use of an adjuvant. [11] Wide local excision followed by endoprosthesis reconstruction is an option to further decrease the recurrence rate to just around 2%. [11] But it is associated with higher surgical risk and all sorts of implant complications such as infection, loosening, and osteolysis in the future. Moreover, in the proximal humerus, reattachment of the rotator cuff to the metallic implant is a challenging issue. As GCT is, in general, a benign disease, the extent of surgery should strike a balance between surgical morbidity, functional loss, and the risk of local recurrence. Our patient was a good example of this dilemma. Before denosumab treatment, salvaging his shoulder joint would be extremely difficult because the cortical bone stock was too little. Fortunately, he had the progressive formation of bone after denosumab as shown on X-ray. From the literature, around 65-80% of the tumor would show a significantly increased fibro-osseous tissue or new bone formation after denosumab treatment. [9],[12] In some specimens, there was even a smooth transition from the osteoid formation in the tumor center, to irregular woven bone in the periphery of the tumor, and to the normal lamellar bone in the normal surrounding tissue.
Our patient represented a good responder to denosumab treatment. However, stromal cells were still present in the excised tumor tissue. Mak et al. examined GCT specimens in an in-vitro study and compared the stromal cell proliferation rate of patients treated and not treated by denosumab. [13] It was shown that a specimen from patients who had completed denosumab treatment still showed the presence of stromal cells. The stromal cells would also continue to proliferate, albeit rather slowly, once they were no longer exposed to denosumab. It is clear from this study that denosumab cannot be used as the sole treatment for GCT. It can only be considered as an adjunct to definitive operative treatment.
However, this patient also illustrated an unexpected problem after denosumab treatment of GCT. The cellular response to denosumab was so prominent that the consistency of the tumor changed. Intraoperatively, the tumor tissue was hard. It could not be removed by curettage. Instead, rongeur and scalpel were needed to remove the tumor tissue in a piecemeal fashion. The usual difference in texture between tumor tissue and bony cortex became blurred. This posed three surgical difficulties. First, there is an increased chance of perforation of subchondral bone resulting in an intra-articular fracture. Classically, GCT has a fleshy and soft texture, which can be easily distinguished from bone. Traditionally, it is removed by curettage, followed by a high-speed burr to remove residual tumor tissue that is still attaching to the surrounding hard cortical and subchondral bone. However, when the tumor tissue becomes hard, sharp instruments need to be used. But the surgeon actually has little tactile feeling to determine how far and how much tissue should be removed. If one goes too far, the sharp instruments can easily cut through the subchondral bone and enter the shoulder joint. If unnoticed, cement may enter the joint during the subsequent cementation procedure. Second, there is an increased chance of neurovascular injury. The axillary artery and brachial plexus are located medial to the proximal humerus. Usually, the cortical bone acts as a good barrier to protect these structures as long as the curettage procedure is done in the intraosseous compartment. However, when a sharp instrument is used, and the cortex is thin, it may penetrate the cortex and cut the neurovascular structure. Third, there may be a risk of increased recurrence. When surgeons sense the danger of intra-articular perforation and neurovascular injury, the natural tendency would be to adopt a more conservative approach to tumor eradication. As a result, there may be tumor tissue left on the surface of the endosteal bone. The risk of residual disease may be increased. Fortunately, our patient did not have any evidence of tumor recurrence on the latest follow-up.
 Conclusion Conclusion | |  |
We propose that surgical excision alone remains the treatment of choice for simple GCT cases in which a standard extended curettage is feasible. But for advanced GCTs that cause significant thinning of surrounding bone and difficulty in salvaging the neighboring joint, trial of denosumab therapy is worthwhile because, in good responders, satisfactory clinical results can be achieved. Denosumab has the potential to eliminate osteolysis and allow time for some local bone reconstitution before operation. The drug is usually well-tolerated. But surgeons should also know the potential difficulties in operating on patients treated with denosumab. They should also be aware that only around 65-80% of patients may show response to denosumab. Up to the current moment, there is no large scale study in the literature to demonstrate any benefit of this drug in reducing local recurrence in the long-term.
 References References | |  |
| 1. | Mendenhall WM, Zlotecki RA, Scarborough MT, Gibbs CP, Mendenhall NP. Giant cell tumor of bone. Am J Clin Oncol 2006;29:96-9.  |
| 2. | Turcotte RE, Wunder JS, Isler MH, Bell RS, Schachar N, Masri BA, et al. Giant cell tumor of long bone: A Canadian sarcoma group study. Clin Orthop Relat Res 2002;397:248-58.  |
| 3. | Thomas DM, Skubitz KM. Giant cell tumour of bone. Curr Opin Oncol 2009;21:338-44.  |
| 4. | Brown JE, Coleman RE. Denosumab in patients with cancer-a surgical strike against the osteoclast. Nat Rev Clin Oncol 2012;9:110-8.  |
| 5. | Thomas DM. RANKL, denosumab, and giant cell tumor of bone. Curr Opin Oncol 2012;24:397-403.  |
| 6. | Rachner TD, Hadji P, Hofbauer LC. Novel therapies in benign and malignant bone diseases. Pharmacol Ther 2012;134:338-44.  |
| 7. | McClung MR, Lewiecki EM, Cohen SB, Bolognese MA, Woodson GC, Moffett AH, et al. Denosumab in postmenopausal women with low bone mineral density. N Engl J Med 2006;354:821-31.  |
| 8. | Smith MR, Saad F, Coleman R, Shore N, Fizazi K, Tombal B, et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: Results of a phase 3, randomised, placebo-controlled trial. Lancet 2012;379:39-46.  |
| 9. | Thomas D, Henshaw R, Skubitz K, Chawla S, Staddon A, Blay JY, et al. Denosumab in patients with giant-cell tumour of bone: An open-label, phase 2 study. Lancet Oncol 2010;11:275-80.  |
| 10. | Vult von Steyern F, Bauer HC, Trovik C, Kivioja A, Bergh P, Holmberg Jörgensen P, et al. Treatment of local recurrences of giant cell tumour in long bones after curettage and cementing. A Scandinavian sarcoma group study. J Bone Joint Surg Br 2006;88:531-5.  |
| 11. | Arbeitsgemeinschaft Knochentumoren. Local recurrence of giant cell tumor of bone after intralesional treatment with and without adjuvant therapy. J Bone Joint Surg Am 2008;90:1060-7.  |
| 12. | Branstetter DG, Nelson SD, Manivel JC, Blay JY, Chawla S, Thomas DM, et al. Denosumab induces tumor reduction and bone formation in patients with giant-cell tumor of bone. Clin Cancer Res 2012;18:4415-24.  |
| 13. | Mak IW, Evaniew N, Popovic S, Tozer R, Ghert M. A translational study of the neoplastic cells of giant cell tumor of bone following neoadjuvant denosumab. J Bone Joint Surg Am 2014;96:e127.  |
[Figure 1], [Figure 2], [Figure 3], [Figure 4], [Figure 5]
|
