| |


 |
| Year : 2016 | Volume
: 10
| Issue : 2 | Page : 59-66 |
|
|
|
|
|
ORIGINAL ARTICLE Accuracy of patient-specific instrumentation in anatomic and reverse total shoulder arthroplasty
Richard James Dallalana1, Ryan A McMahon2, Ben East3, Liam Geraghty4
1 Department of Orthopaedics, Western Health; Park Clinic Orthopaedics, St. Vincent's Hospital, Melbourne, Victoria, Australia
2 Department of Orthopaedics, St. Vincent's Hospital, Melbourne, Victoria, Australia
3 Department of Orthopaedics, John Hunter Hospital, Newcastle, Australia
4 Department of Orthopaedics, Western Health, Melbourne, Victoria, Australia
Correspondence Address:
Richard James Dallalana
Suite 71/166 Gipps Street, East Melbourne, Victoria 3002
Australia
 Source of Support: None, Conflict of Interest: None  | Check |
DOI: 10.4103/0973-6042.180717
 |
|
|
|
| Date of Web Publication | 20-Apr-2016 |
 Abstract Abstract | | |
Purpose: Glenoid component malposition is associated with poor function and early failure of both anatomic and reverse total shoulder arthroplasty. Glenoid positioning is challenging particularly in the setting of bone loss or deformity. Recently, the use of computer assistance has been shown to reduce implantation error. The aim of this study is to evaluate the accuracy of patient-specific instrumentation in cases of anatomic and reverse shoulder replacement in vivo.
Methods: Twenty patients underwent total shoulder arthroplasty using a computed tomography (CT)-based patient-specific instrumentation (PSI) system, ten anatomic and ten reverse. Preoperative three-dimensional digital templating of glenoid component position was undertaken and surgery then performed using a custom-made guide. Postoperative CT scans were used to compare final implanted component position to the preoperatively planned position in the same patient.
Results: Final component position and orientation closely reflected the preoperatively templated position. Mean deviation in the glenoid version from planned was 1.8° ±1.9° (range, 0.1°-7.3°). Mean deviation in inclination was 1.3° ±1.0° (range, 0.2°-4.5°). Mean deviation in position on the glenoid face was 0.5 ± 0.3 mm (range, 0.0-1.3 mm) in the anteroposterior plane and 0.8 ± 0.5 mm (range, 0.0-1.9 mm) in the superoinferior plane. Actual achieved version was within 7° of neutral in all cases except for one where it was deliberately planned to be outside of this range.
Conclusion: PSI in both anatomic and reverse shoulder arthroplasty is highly accurate in guiding glenoid component implantation in vivo. The system can reliably correct bony deformity.
Keywords: Custom guide, digital templating, glenoid malposition, patient-specific, shoulder arthroplasty, three-dimensional planning
How to cite this article:
Dallalana RJ, McMahon RA, East B, Geraghty L. Accuracy of patient-specific instrumentation in anatomic and reverse total shoulder arthroplasty. Int J Shoulder Surg 2016;10:59-66 |
 Introduction Introduction | |  |
Both anatomic and reverse total shoulder replacement (TSR) are highly successful treatment options for degenerative conditions of the shoulder. [1],[2],[3] Technically, the most challenging aspect of the procedure is the insertion of the glenoid component and issues relating to the glenoid are a common cause of poor function or early revision surgery. [4],[5],[6] Secure primary fixation and optimal position and orientation of the glenoid component are fundamental to the success of total shoulder arthroplasty, yet inaccuracy in its implantation has long been tolerated due to inherent surgical difficulties.
Malposition of the glenoid component in primary anatomic shoulder replacement is associated with increased shear forces and wear, early loosening and revision, and instability. [7],[8],[9],[10],[11] Poor glenosphere position in reverse shoulder replacement is linked to reduced range of motion from impingement, increased scapular notching, instability, and loosening leading to early revision or catastrophic failure of the component. [1],[12],[13],[14],[15]
The process of positioning the glenoid component accurately is challenging due to the limited exposure available, the degree of bony deformity often present, and the lack of reliable landmarks to orientation. Examination of glenoid position achieved with standard instrumentation reveals significant error, even in the hands of experienced shoulder surgeons. [16],[17],[18],[19]
Various systems have been developed to assist glenoid implantation including the use of electromagnetic tracking devices [17] and computer navigation using reflective markers. [16],[18],[20] These devices have been shown to improve accuracy, however, are hampered by an increase in surgical time, issues related to pin placement, and lack of reliability. More recently the use of patient-specific instrumentation (PSI) systems using custom-made guides constructed from computed tomography (CT)-based three-dimensional (3D) preoperative computer templating has grown in popularity. Improvement in glenoid positioning using PSI compared to standard instrumentation has been shown in other studies. [21],[22],[23],[24]
Most work published on this technology is cadaver-based. The purpose of this study is to quantify the accuracy of PSI in both anatomic and reverse shoulder replacement in the live surgical setting where more sources of error exist and where it is most relevant. We examine the concordance between the measured position of the actual glenoid component on postoperative CT scan and its position planned preoperatively using 3D computer templating.
Confidence in the on-going use of PSI systems to guide glenoid implantation is increased with validation of their accuracy. The use of PSI theoretically stands to improve both function and survivorship outcomes through more accurate component placement.
 Methods Methods | |  |
The study was approved by the Melbourne Health Human Research Ethics Committee and the Institutional Review Board at Western Health, Melbourne, Australia. Twenty sequential patients were drawn from our institution waiting list and from the private practice of the senior author. All underwent PSI-assisted total shoulder arthroplasty surgery, ten anatomic and ten reverse, by the same surgeon (Richard James Dallalana) using the DJO Surgical Match Point System (DJO, Austin, TX, USA).
Preoperative CT scanning was carried out according to specific parameters: Sixteen-slice multi-detector CT scanner (SOMATOM Emotion, Siemens Medical Solutions USA, Inc.); supine position with arms at the side and scapula in a neutral position; no gantry tilt, 120 kV, automatically set mAs, 1.25 mm slice thickness, 0.625 mm. Slice increment, 512 × 512 matrix and 25 cm × 25 cm field of view encompassing the scapular and humeral head. Desired glenoid component position for each case was planned using SurgiCase Connect software (Materialise, Leuven, Belgium) [Figure 1]a and b. This system requires full determination of glenoid component positioning by the operating surgeon on screen. No interaction with engineers or other external input during this planning phase takes place.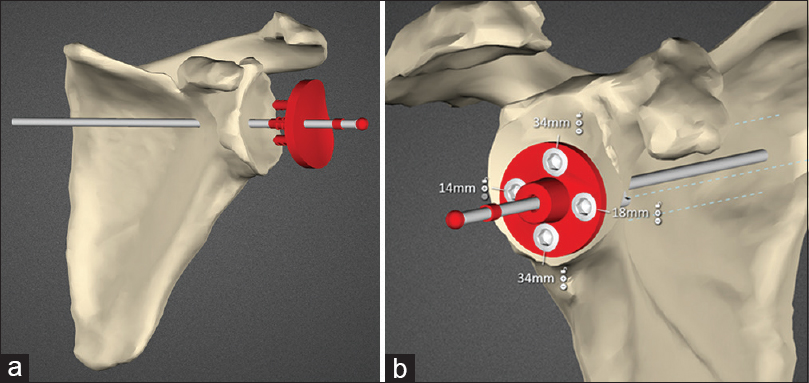 | Figure 1: (a) Planning screenshot showing the patient scapula and the anatomic glenoid component being positioned. (b) Planning screenshot showing reverse baseplate with locking screws and lengths seated on reamed bone
Click here to view |
Reference was made to the transverse scapular axis as previously described. [25],[26],[27] For reverse cases neutral version and approximately 7° of inferior angulation with respect to a normal glenoid face were chosen, unless the nature of individual deformity required deliberate deviation from these parameters. For anatomic cases, neutral version and neutral inclination were selected unless bone stock dictated otherwise.
From the final planned position, a custom-made guide is constructed and used intra-operatively to direct the glenoid pilot drill start position and trajectory [Figure 2]. Postoperatively, all twenty study participants underwent repeat CT scanning with metal artifact subtraction from which the position and angle of the actual component could be measured and compared to the intended position and angle on the preoperative surgical plan.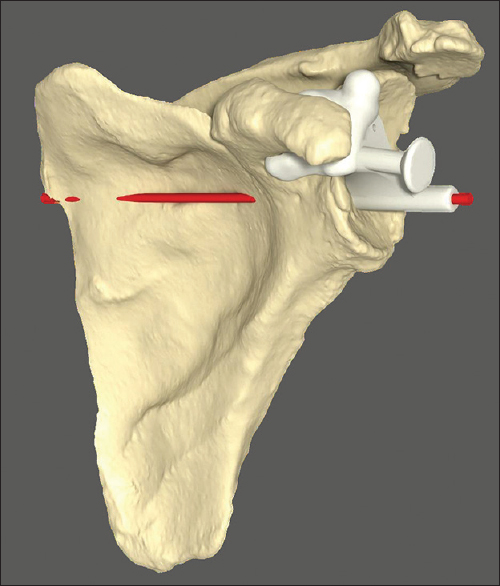 | Figure 2: Custom-made guide seated on scapular model. Intended trajectory of initial guide drill shown in red
Click here to view |
Surgical technique
The DJO Surgical Reverse Shoulder Prosthesis (DJO, Austin, TX, USA) and DJO Surgical Turon Modular Shoulder System (DJO, Austin, TX, USA) were utilized.
All were performed through a standard deltopectoral approach. Biceps tenotomy is required to allow adequate clearance of soft tissue from the supraglenoid tubercle to the base of the coracoid. The custom guide has two small flanges which pass around the anterior and posterior aspects of the coracoid base requiring this area to be free of any soft tissue. The remaining part of the guide rests on the glenoid face [Figure 3]. Residual articular cartilage is removed if it impedes secure seating of the device. Osteophytes are retained to ensure the guide rests on a completely conforming bony surface. Once the initial drill is passed the custom guide is removed and further steps performed according to the standard technique.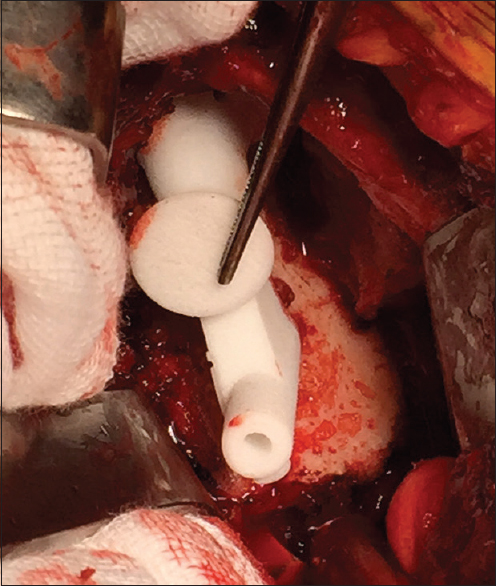 | Figure 3: Intra-operative view of guide for reverse shoulder prosthesis seated on glenoid face
Click here to view |
The depth of reaming is controlled manually by referencing the appearance of the reamed glenoid bone during this step with the appearance of the final "reamed" computer image with the prosthesis subtracted. Locking screws for the reverse are perpendicular to the baseplate, and thus, direction is predetermined. An estimation of screw length is shown on the preoperative plan however each length is individually confirmed by direct measurement. Manual adjustment is possible with respect to the final rotational position of the baseplate, limited by how quickly it tightens during insertion. The guide for the anatomic pegged glenoid has a dual cannulation to control precisely for component rotation [Figure 4].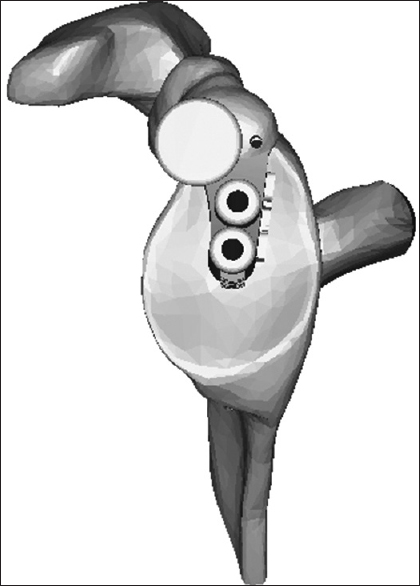 | Figure 4: Diagram of guide for the Turon (anatomic prosthesis) depicting dual cannulation for rotational control
Click here to view |
The custom-made guide alone was used to direct glenoid positioning in all cases without the use of standard instruments. Two reverse cases required primary glenoid bone grafting to allow correction of extreme retroversion of approximately 45° [Figure 5].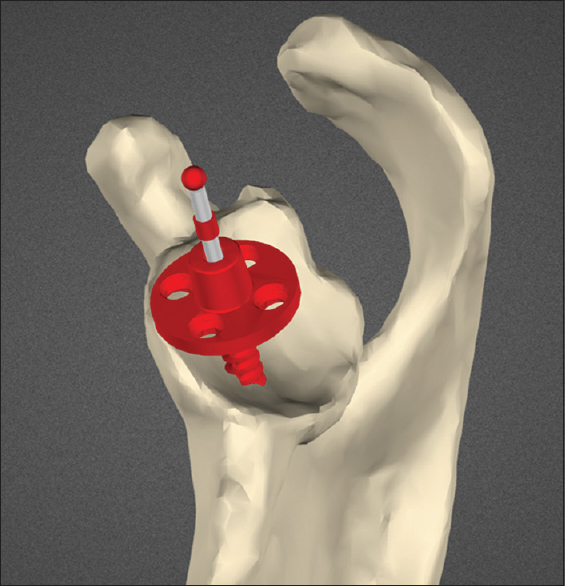 | Figure 5: Planning screenshot demonstrating severe glenoid retroversion and posterior defect requiring grafting
Click here to view |
Comparative measurement technique
Measurements were performed by an unaffiliated university research student (Ryan A. McMahon). Axial and coronal slices on the postoperative CT scans which best visualized the glenoid components were selected from the entire volume data. Version angle was measured with reference to the transverse scapular axis as defined by Friedman et al. [25] Digital markers on the CT images were used as reference points to enable localization of the identical axial plane on the preoperative digital planning images. On the digital plan, version was then measured in the same axial plane to record the intended version for that same patient [Figure 6]a and b. The component inclination was measured with reference to a line from the mid-point of the glenoid component to the trigonum spinae in a coronal slice along the plane of the scapula body, and compared in the same way to the intended angle of inclination on preoperative plan. Deviation between the planned and actual component angulation was recorded in degrees. Position on the glenoid face was compared by measuring the distance from the margins of the glenoid component or from the central peg outline to bony points on the glenoid in both the CT scans and preoperative digital plan, and recorded in mm [Figure 7]a and b.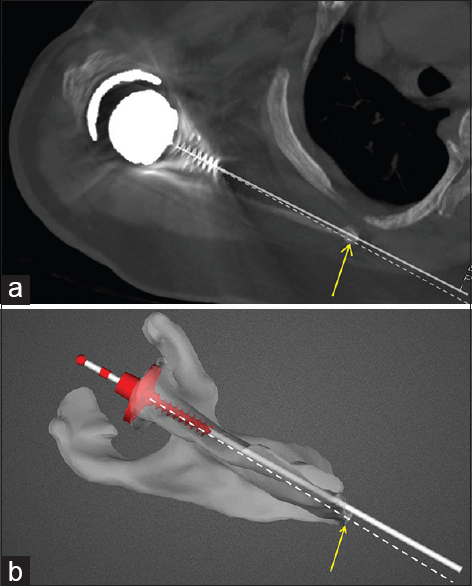 | Figure 6: (a) Version measurement on postoperative computed tomography scan. Bold white line represents actual glenoid component trajectory. Dashed line represents the transverse scapular axis (mid-point of glenoid to medial border scapula). Angle subtended between the two lines being the measured version of the component. (b) Version measurement on preoperative planning image for the same patient as in Figure 6a. Bold white line represents intended glenoid component trajectory. Dashed white line represents the transverse scapular axis. Angle between the two lines being the version of the component with respect to the transverse scapular axis
Click here to view |
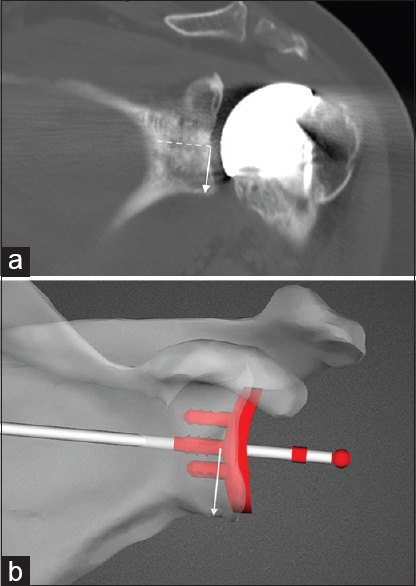 | Figure 7: (a) Measurement of actual component position in the supero-inferior plane on postoperative computed tomography scan. Solid white arrow is perpendicular to the line of the central peg of the glenoid (dashed line). (b) Measurement of intended glenoid position on preoperative planning image for the same patient as in Figure 7a
Click here to view |
Statistical analysis was performed using SPSS 22.0 software (SPSS Inc., Chicago, IL, USA).
 Results Results | |  |
[Table 1] shows the mean of measured deviations in version and inclination. The values listed refer to the mean difference between the angle of glenoid orientation measured on the actual component and that which was planned preoperatively. Deviation from planned component version was 2.6° ± 2.2° (range, 0.2°-7.3°) in anatomic replacement, and 1.1° ± 1.1° (range, 0.1°-4.0°) in reverse cases. For all twenty cases, the mean deviation in component version was 1.8° ± 1.9° (range, 0.1°-7.3°). Deviation in inclination for anatomic replacement was 1.0° ± 0.7° (range, 0.3°-2.7°) and 1.6° ± 1.1° (range, 0.2°-4.5°) for reverse. Mean deviation in inclination for all twenty cases was 1.3° ± 1.0° (range, 0.2°-4.5°). | Table 1: Mean deviation between planned and actual glenoid component version and inclination
Click here to view |
[Table 2] shows the mean deviation in position on the glenoid face between actual and planned. Mean deviation from planned component position for all twenty cases in the anteroposterior plane was 0.5 ± 0.3 mm (range, 0.0-1.3 mm), and 0.8 ± 0.5 mm (range, 0.0-1.9 mm) in the superoinferior plane.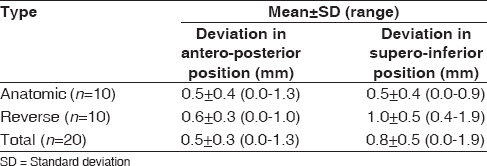 | Table 2: Mean deviation between planned and actual glenoid component position
Click here to view |
[Table 3] lists the planned and actual component version and inclination measurements for each patient, as well as the difference calculated between planned and actual. Final version achieved was within 7° of neutral for 19 of the 20 cases with the one exception being for a case planned preoperatively to be outside of this range.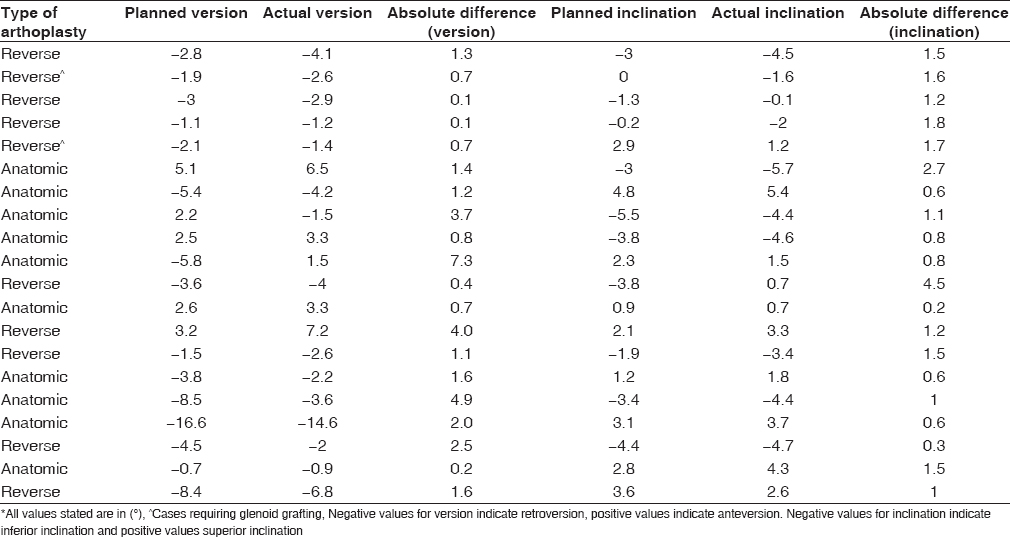 | Table 3: Measured values of component version and inclination for all twenty cases and the calculated difference between planned and actual*
Click here to view |
The custom guide studied was unobtrusive and easily positioned once soft tissue was appropriately resected from the coracoid base. There were no specific complications related to its use.
 Discussion Discussion | |  |
For some time, a need has existed to improve the accuracy of glenoid component placement in total shoulder arthroplasty. Glenoid component implantation is challenging and malposition is common due to inherent technical difficulties.
PSI systems are being shown to improve the accuracy of glenoid positioning and with time, it is expected that outcomes for patients will likewise be improved. Validation of the accuracy of such systems in live surgical cases is required to engender confidence in their routine use as a surgical tool, particularly during cases of glenoid bone deformity when standard guides cannot be used.
Early failure of anatomic replacement is frequently associated with glenoid loosening. This can result from inadequate component seating, eccentric loading, poor cementation or improper component orientation, particularly excessive residual retroversion. [28],[29],[30] It has been noted that retroversion of >15° is associated with early prosthetic wear, instability, and failure. [31] Glenoid component malposition is linked to increased radiolucent lines and poor clinical performance. [19],[32]
Reverse TSR function and range of motion are influenced by glenosphere position and angle. Deviations of the glenoid baseplate by 5 mm from the optimal position can reduce the range of motion. [13] Version error can lead to instability. [13] Inclination error can lead to failure from excessive superior tilt. [33],[34] Sound initial fixation in solid bone is crucial to confer adequate stability to allow bony on-growth and prevent early loosening. [35]
Glenoid malposition is noted to be common even in what would be considered straightforward cases. With standard instrumentation significant version and position error have been reported. [17],[18] CT examination of implant position in cases performed by experienced shoulder surgeons has shown a high rate of version error and other faults. [16],[17],[18],[19]
The challenges faced in glenoid component implantation are multiple. The difficulty with exposure due to joint contracture, shortening, anatomical variation, and body habitus are well recognized. In the setting of bone erosion and deformity problems leading to inaccurate placement are compounded. Standard instruments cannot be used, and the ability to convert one's intended glenoid position through manual examination of preoperative scans to an accurate reproduction of that position intra-operatively is limited. There are few reliable bony landmarks to inform of the exact size and orientation of the residual glenoid vault. [36] In addition, the plane of the scapula from which to determine appropriate version and inclination cannot often be reliably appreciated. Extreme erosion requiring primary bone grafting can present an even greater reliance on exact positioning to optimize fixation in the best native bone available.
PSI can improve glenoid positioning while addressing many of the shortcomings of earlier navigation using reflective markers. [16],[17],[18],[20],[37] Levy et al. [38] looked at the drill paths for the anticipated reverse baseplate position in cadaveric scapulae and showed a high degree of accuracy of the PSI system to reproduce the intended path planned preoperatively. Likewise, Throckmorton et al. [21] demonstrated improved accuracy using 3D planning techniques coupled with a patient-specific guide system compared to implantation with standard instruments in seventy cadavers. In a study using normal cadaver specimens by Walch et al. [39] the use of a PSI system resulted in faithful reproduction of the surgical plan when measuring guide pin position.
Additional sources of potential error, however, are introduced in the live surgical setting which may reduce the accuracy of these systems. The difficulty with access due to joint contracture, inadequate soft tissue release, and a smaller exposure may impede the straight passage of the initial drill and reamer. The intra-operative guide may be seated on bone improperly if soft tissue clearance from the relevant region of the glenoid is compromised by bleeding or time constraints. Levels of deformity being addressed are generally greater.
Hendel et al. [22] compared the outcomes of glenoid positioning in anatomic TSR with and without the use of PSI in a randomized study of 31 cases. They demonstrated that PSI was significantly better than standard instruments, particularly with more severe deformity. Iannotti et al. [24] reported accurate reproduction of preoperative planning using a reusable patient-specific instrument and also attested to the utility of 3D planning and templating as a tool even without the intra-operative instrument. A study of 15 cases including both anatomic and reverse replacements by Throckmorton et al. [40] using postoperative CT measurement showed a deviation of 4° from planned version and of 5° from planned inclination, with a starting position variation of 2 mm. They used computer scripts to measure differences from the "best-fit" of pre- and post-operative scapular reconstructions, rather than direct manual measurement of available images as performed in our study. It is not known whether one measurement technique confers an advantage over another. Heylen et al. [23] noted reduced variability in glenoid component inclination using PSI in an X-ray based review. Trouilloud et al. [41] in five reverse arthroplasties and Suero et al. [37] in a series of ten TSRs showed good reproducibility of the preoperative surgical plan using CT-based PSI systems.
Our study is a live surgical series of twenty cases including both anatomic and reverse replacement performed with PSI for the glenoid. Position on preoperative digital templating was compared to measurements made on postoperative CT scan of the same patient. For the whole group of twenty arthroplasties, we noted a mean deviation of 1.8° in version from what was planned, and 1.3° for inclination [Table 1]. Mean deviation of component position on the glenoid face was 0.5 mm and 0.8 mm in the A-P and S-I directions, respectively [Table 2]. While as yet there is no clear industry benchmark to which these results can be compared, the absolute values are small and compare favorably with the few similar studies available in the published literature. The range of deviation was narrow with a single outlier of 7.3° in version, still at an acceptable level for live case surgery and considering also a potential contribution from measurement error. The deviations in position and angle noted were random and not confined to a single direction of error [Table 3]. No significant difference in accuracy of the system comparing anatomic to reverse replacement was observed. No other instruments were used to direct the initial pilot drill and no attempt to use other reference points intra-operatively to reconsider the drill trajectory were made. Our results demonstrate a high degree of concordance between preoperative planning and the final implanted component position, reflecting the accuracy of the system studied in its ability to reproduce the surgical plan.
The PSI system studied was effective in correcting deformity of the arthritic glenoid. Most of the cases in our series exhibited a degree of anatomical deformity of varying severity and typically characterized by glenoid retroversion. Standard instrumentation designed for relatively normal anatomy is ineffective in these situations. The final version achieved in each of the twenty patients in our cohort is listed in [Table 3], where version is measured with respect to the transverse axis of the scapula. Nineteen of the twenty cases had a measured final component version within 7° of neutral. The exception was of an anatomic replacement resting at 14.6° retroversion. This was planned preoperatively to be at 16.6° retroversion due to severe posterior erosion (22° retroverted) and need to avoid excessive high-side reaming in that case. It was a glenoid poorly suited to a nonaugmented pegged glenoid component but highlights the advantage of using the accuracy of PSI to proceed in a marginal situation.
Cases of substantial bone loss with severe retroversion or other deformity treated with reverse arthroplasty and primary bone grafting are particularly challenging [42],[43],[44] and it is in these cases where the use of PSI may be of greatest advantage. Erosion frequently occurs in both the transverse and coronal planes. [45] The system studied facilitated correction of orientation in these complex multiplanar deformities while achieving strong primary baseplate fixation. We noted the central screw of the reverse prosthesis could be reliably placed in the highest quality bone available at the medial end of the vault, along a line parallel to the scapular spine. This location has been shown to confer a high level of stability of fixation. [46] Finding this location by feel or by manual interpretation of CT scanning is unreliable while the start point on a deformed face is always hard to judge. It is of particular importance in cases requiring bone grafting that central screw fixation is optimized when a low percentage of native bony contact with the back of the baseplate exists. It is worthy of note that final component alignment in the two cases requiring bone grafting showed the same degree of accuracy as the nongrafted cases, both close to neutral version and with minimal deviation from preoperative plan [Table 3].
Historically, TSRs have enjoyed relatively good survivorship and functional outcomes. [1],[2],[3],[14] The extent of use therefore of a new technology comes into focus, and the issue may become one of how high the bar needs to be set with regards accuracy of component implantation. It has been demonstrated that the maximum benefit of PSI systems may be in cases of more severe glenoid deformity. [22] On the other hand, a high incidence of glenoid positioning error is known to occur across all cases even in experienced hands, [19] a situation thus potentially improved by the use of PSI in every case rather than only selected ones. In our experience, it has become a routine to use it in all patients and adds a welcome degree of predictability in the surgical outcome even in less challenging cases. Costs of this technology may be recouped on a community basis if revision rates are lowered and patient function is higher, however this remains to be determined.
There is a learning curve with the use of any new technology. The 3D templating software studied allows for complete user control with infinite adjustment possible in real-time placing the onus on the operating surgeon to draw on an appropriate skillset during the planning phase. The principles of prosthetic reconstruction of the glenoid then need to be applied to the unfamiliar scenario of a computer generated image. Good surgical technique now needs to be good "virtual" surgical technique. There are some pitfalls at this planning stage such as the temptation to be over-zealous during asymmetric reaming to correct retroversion when one would perhaps be more conservative in the live surgical scenario. There is also a tendency on screen to excessively tilt the reverse baseplate inferiorly at the cost of bone stock. An undersized anatomic glenoid component visually can appear acceptable on screen but in practice would not be ideal. A poorly planned procedure will be reproduced as such intra-operatively, particularly as we have validated the ability of the system to accurately replicate that plan. Specific training in the use of the planning software is recommended before its use.
An advantage of PSI is the transfer of the process of planning glenoid component position from the operating theater to the office or home where there is no time constraint and here it can also serve as an effective educational tool. A detailed discussion about the decision-making process regarding glenoid positioning can be held while the procedure is performed on screen, rather than in the operating theater.
With 3D preoperative planning deformity can be defined with greater precision. This alone improves the accuracy of implantation. [24] The need for grafting can be preempted with some certainty. Severe deformity requiring a custom prosthesis or precluding the use of a glenoid component at all can be determined ahead of time. We have shown that one may have confidence intra-operatively that the custom guide will direct the initial drill to the preoperatively planned position and trajectory, removing delay and potential uncertainty during this phase of the operation. This reproducibility may be of even greater benefit for low volume shoulder arthroplasty surgeons. Ongoing clinical trials may ultimately be able to show an effect on prosthesis longevity and clinical function with the use of this technology.
 Conclusion Conclusion | |  |
PSI can reliably and accurately guide glenoid component implantation in anatomic and reverse shoulder replacement in vivo. The system can reliably correct deformity and enable glenoid placement in optimal orientation in all cases including where retroversion or other deformity is present.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
 References References | |  |
| 1. | Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am 2006;88:1742-7.  |
| 2. | Matsen FA 3 rd . Early effectiveness of shoulder arthroplasty for patients who have primary glenohumeral degenerative joint disease. J Bone Joint Surg Am 1996;78:260-4.  |
| 3. | Sperling JW, Cofield RH, Rowland CM. Neer hemiarthroplasty and Neer total shoulder arthroplasty in patients fifty years old or less. Long-term results. J Bone Joint Surg Am 1998;80:464-73.  |
| 4. | Antuna SA, Sperling JW, Cofield RH, Rowland CM. Glenoid revision surgery after total shoulder arthroplasty. J Shoulder Elbow Surg 2001;10:217-24.  |
| 5. | Boileau P, Melis B, Duperron D, Moineau G, Rumian AP, Han Y. Revision surgery of reverse shoulder arthroplasty. J Shoulder Elbow Surg 2013;22:1359-70.  |
| 6. | Wirth MA, Rockwood CA Jr. Complications of shoulder arthroplasty. Clin Orthop Relat Res 1994;307:47-69.  |
| 7. | Hopkins AR, Hansen UN, Amis AA, Emery R. The effects of glenoid component alignment variations on cement mantle stresses in total shoulder arthroplasty. J Shoulder Elbow Surg 2004;13:668-75.  |
| 8. | Karduna AR, Williams GR, Iannotti JP, Williams JL. Total shoulder arthroplasty biomechanics: A study of the forces and strains at the glenoid component. J Biomech Eng 1998;120:92-9.  |
| 9. | Moeckel BH, Altchek DW, Warren RF, Wickiewicz TL, Dines DM. Instability of the shoulder after arthroplasty. J Bone Joint Surg Am 1993;75:492-7.  |
| 10. | Norris TR, Iannotti JP. Functional outcome after shoulder arthroplasty for primary osteoarthritis: A multicenter study. J Shoulder Elbow Surg 2002;11:130-5.  |
| 11. | Nyffeler RW, Sheikh R, Atkinson TS, Jacob HA, Favre P, Gerber C. Effects of glenoid component version on humeral head displacement and joint reaction forces: An experimental study. J Shoulder Elbow Surg 2006;15:625-9.  |
| 12. | Favard L, Levigne C, Nerot C, Gerber C, De Wilde L, Mole D. Reverse prostheses in arthropathies with cuff tear: Are survivorship and function maintained over time? Clin Orthop Relat Res 2011;469:2469-75.  |
| 13. | Favre P, Sussmann PS, Gerber C. The effect of component positioning on intrinsic stability of the reverse shoulder arthroplasty 2010;19:550-6.  |
| 14. | Gutiérrez S, Comiskey CA 4 th , Luo ZP, Pupello DR, Frankle MA. Range of impingement-free abduction and adduction deficit after reverse shoulder arthroplasty. Hierarchy of surgical and implant-design-related factors. J Bone Joint Surg Am 2008;90:2606-15.  |
| 15. | Nyffeler RW, Werner CM, Gerber C. Biomechanical relevance of glenoid component positioning in the reverse Delta III total shoulder prosthesis. J Shoulder Elbow Surg 2005;14:524-8.  |
| 16. | Kircher J, Wiedemann M, Magosch P, Lichtenberg S, Habermeyer P. Improved accuracy of glenoid positioning in total shoulder arthroplasty with intraoperative navigation: A prospective-randomized clinical study. J Shoulder Elbow Surg 2009;18:515-20.  |
| 17. | Nguyen D, Ferreira LM, Brownhill JR, King GJ, Drosdowech DS, Faber KJ, et al. Improved accuracy of computer assisted glenoid implantation in total shoulder arthroplasty: An in-vitro randomized controlled trial. J Shoulder Elbow Surg 2009;18:907-14.  |
| 18. | Verborgt O, De Smedt T, Vanhees M, Clockaerts S, Parizel PM, Van Glabbeek F. Accuracy of placement of the glenoid component in reversed shoulder arthroplasty with and without navigation. J Shoulder Elbow Surg 2011;20:21-6.  |
| 19. | Gregory TM, Sankey A, Augereau B, Vandenbussche E, Amis A, Emery R, et al. Accuracy of glenoid component placement in total shoulder arthroplasty and its effect on clinical and radiological outcome in a retrospective, longitudinal, monocentric open study. PLoS One 2013;8:e75791.  |
| 20. | Edwards TB, Gartsman GM, O'Connor DP, Sarin VK. Safety and utility of computer-aided shoulder arthroplasty. J Shoulder Elbow Surg 2008;17:503-8.  |
| 21. | Throckmorton TW, Gulotta LV, Bonnarens FO, Wright SA, Hartzell JL, Rozzi WB, et al. Patient-specific targeting guides compared with traditional instrumentation for glenoid component placement in shoulder arthroplasty: A multi-surgeon study in 70 arthritic cadaver specimens. J Shoulder Elbow Surg 2015;24:965-71.  |
| 22. | Hendel MD, Bryan JA, Barsoum WK, Rodriguez EJ, Brems JJ, Evans PJ, et al. Comparison of patient-specific instruments with standard surgical instruments in determining glenoid component position: A randomized prospective clinical trial. J Bone Joint Surg Am 2012;94:2167-75.  |
| 23. | Heylen S, Van Haver A, Vuylsteke K, Declercq G, Verborgt O. Patient-specific instrument guidance of glenoid component implantation reduces inclination variability in total and reverse shoulder arthroplasty. J Shoulder Elbow Surg 2016;25:186-92.  |
| 24. | Iannotti JP, Weiner S, Rodriguez E, Subhas N, Patterson TE, Jun BJ, et al. Three-dimensional imaging and templating improve glenoid implant positioning. J Bone Joint Surg Am 2015;97:651-8.  |
| 25. | Friedman RJ, Hawthorne KB, Genez BM. The use of computerized tomography in the measurement of glenoid version. J Bone Joint Surg Am 1992;74:1032-7.  |
| 26. | De Wilde LF, Verstraeten T, Speeckaert W, Karelse A. Reliability of the glenoid plane. J Shoulder Elbow Surg 2010;19:414-22.  |
| 27. | Churchill RS, Brems JJ, Kotschi H. Glenoid size, inclination, and version: An anatomic study. J Shoulder Elbow Surg 2001;10:327-32.  |
| 28. | Fox TJ, Cil A, Sperling JW, Sanchez-Sotelo J, Schleck CD, Cofield RH. Survival of the glenoid component in shoulder arthroplasty. J Shoulder Elbow Surg 2009;18:859-63.  |
| 29. | Franta AK, Lenters TR, Mounce D, Neradilek B, Matsen FA 3 rd . The complex characteristics of 282 unsatisfactory shoulder arthroplasties. J Shoulder Elbow Surg 2007;16:555-62.  |
| 30. | Matsen FA 3 rd , Clinton J, Lynch J, Bertelsen A, Richardson ML. Glenoid component failure in total shoulder arthroplasty. J Bone Joint Surg Am 2008;90:885-96.  |
| 31. | Ho JC, Sabesan VJ, Iannotti JP. Glenoid component retroversion is associated with osteolysis. J Bone Joint Surg Am 2013;95:e82.  |
| 32. | Hsu JE, Namdari S, Baron M, Kuntz AF, Abboud JA, Huffman GR, et al. Glenoid perforation with pegged components during total shoulder arthroplasty. Orthopedics 2014;37:e587-91.  [ PUBMED] |
| 33. | Gutiérrez S, Greiwe RM, Frankle MA, Siegal S, Lee WE 3 rd . Biomechanical comparison of component position and hardware failure in the reverse shoulder prosthesis. J Shoulder Elbow Surg 2007;16 3 Suppl:S9-12.  |
| 34. | Molé D, Wein F, Dézaly C, Valenti P, Sirveaux F. Surgical technique: The anterosuperior approach for reverse shoulder arthroplasty. Clin Orthop Relat Res 2011;469:2461-8.  |
| 35. | Hopkins AR, Hansen UN, Bull AM, Emery R, Amis AA. Fixation of the reversed shoulder prosthesis. J Shoulder Elbow Surg 2008;17:974-80.  |
| 36. | Lewis GS, Bryce CD, Davison AC, Hollenbeak CS, Piazza SJ, Armstrong AD. Location of the optimized centerline of the glenoid vault: A comparison of two operative techniques with use of three-dimensional computer modeling. J Bone Joint Surg Am 2010;92:1188-94.  |
| 37. | Suero EM, Citak M, Lo D, Krych AJ, Craig EV, Pearle AD. Use of a custom alignment guide to improve glenoid component position in total shoulder arthroplasty. Knee Surg Sports Traumatol Arthrosc 2013;21:2860-6.  |
| 38. | Levy JC, Everding NG, Frankle MA, Keppler LJ. Accuracy of patient-specific guided glenoid baseplate positioning for reverse shoulder arthroplasty. J Shoulder Elbow Surg 2014;23:1563-7.  |
| 39. | Walch G, Vezeridis PS, Boileau P, Deransart P, Chaoui J. Three-dimensional planning and use of patient-specific guides improve glenoid component position: An in vitro study. J Shoulder Elbow Surg 2015;24:302-9.  |
| 40. | Throckmorton TW, Vogt W, Wasmaier J, Hurst JM, Frostick S, Sperling JW. Patient-specific targeting guides for glenoid component placement in shoulder arthroplasty: Technique and initial clinical experience. Tech Shoulder Elbow Surg 2014;15:103-8.  |
| 41. | Trouilloud P, Gonzalvez M, Martz P, Charles H, Handelberg F, Nyffeler RW, et al. Duocentric® reversed shoulder prosthesis and Personal Fit® templates: Innovative strategies to optimize prosthesis positioning and prevent scapular notching. Eur J Orthop Surg Traumatol 2014;24:483-95.  |
| 42. | Frankle MA, Teramoto A, Luo ZP, Levy JC, Pupello D. Glenoid morphology in reverse shoulder arthroplasty: Classification and surgical implications. J Shoulder Elbow Surg 2009;18:874-85.  |
| 43. | Hill JM, Norris TR. Long-term results of total shoulder arthroplasty following bone-grafting of the glenoid. J Bone Joint Surg Am 2001;83-A:877-83.  |
| 44. | Iannotti JP, Greeson C, Downing D, Sabesan V, Bryan JA. Effect of glenoid deformity on glenoid component placement in primary shoulder arthroplasty. J Shoulder Elbow Surg 2012;21:48-55.  |
| 45. | Habermeyer P, Magosch P, Luz V, Lichtenberg S. Three-dimensional glenoid deformity in patients with osteoarthritis: A radiographic analysis. J Bone Joint Surg Am 2006;88:1301-7.  |
| 46. | Klein SM, Dunning P, Mulieri P, Pupello D, Downes K, Frankle MA. Effects of acquired glenoid bone defects on surgical technique and clinical outcomes in reverse shoulder arthroplasty. J Bone Joint Surg Am 2010;92:1144-54.  |
[Figure 1], [Figure 2], [Figure 3], [Figure 4], [Figure 5], [Figure 6], [Figure 7]
[Table 1], [Table 2], [Table 3]
|
