|
 
 |
| ORIGINAL ARTICLE |
|
| Year : 2013 | Volume
: 1
| Issue : 1 | Page : 36-40 |
|
Relationship between peripheral neuropathy and antiretroviral drugs used in the management of adult human immunodeficiency virus patients
Simeon A Nwabueze1, Ngozi N Joe-Ikechebelu2, Ifeoma A Modebe1, Prosper O. U. Adogu1, Prince U Ele3
1 Department of Community Medicine, Nnamdi Azikiwe University/Teaching Hospital, Nnewi Anambra State, Nigeria
2 Department of Community Medicine, Anambra State University/Teaching Hospital, Awka, Anambra State, Nigeria
3 Department of Internal Medicine, Nnamdi Azikiwe University/Teaching Hospital, Nnewi Anambra State, Nigeria
| Date of Acceptance | 24-Aug-2012 |
| Date of Web Publication | 16-Aug-2013 |
Correspondence Address:
Simeon A Nwabueze
Department of Community Medicine, Nnamdi Azikiwe University/Teaching Hospital, Nnewi, Anambra State
Nigeria
 Source of Support: Research carried out from authors personal
resources,, Conflict of Interest: The Authors hereby declare that there
is no conflict of interest envisaged in course of this research, article
review and publication.  | Check |
 
Introduction: Antiretroviral (ARV) drugs have some associated adverse events that may jeopardize confidence in their safety and alter patient adherence to ARV therapy. Various forms of peripheral neuropathy are associated with human immunodeficiency virus (HIV) infection and its management, and these occur at various stages of HIV disease. This study is aimed at exploring the relationship between peripheral neuropathy and ARV drugs used in the management of adult HIV patients who access care in Nnamdi Azikiwe University Teaching Hospital (NAUTH) Nnewi, a tertiary health facility in South-East Nigeria. Materials and Methods: The study is a review of 7,280 ARV-experienced adult HIV-positive patients' medical records at NAUTH Nnewi, between July 2005 and July 2009. The adverse events electronic data base was searched, 156 patients with reported adverse events were identified and all data of 52 enrollees with peripheral neuropathies (PN) who met the inclusion criteria were reviewed. Results: Median duration of treatment with ARV drugs was 42 weeks. The prevalence of neuropathy was high among the widowed females and those greater than 40 years (P < 0.05, P < 0.01, respectively). More PN were found among subjects on offending drugs than among those on alternate drugs (P < 0.005). All subjects with co-morbidities had peripheral neuropathy, while only 27.8% of those without co-morbidity had the neuropathy (P < 0.001). Conclusion: If co-morbidities are treated in HIV patients, offending drugs such as stavudine, replaced with alternatives like Tenofovir in the management of their medical condition and socio-demographic variables considered in the selection of treatment modalities, the incidence of peripheral neuropathy among HIV patients on ARV drugs will markedly reduce. Keywords: Antiretroviral drugs, human immunodeficiency virus/acquired immune deficiency syndrome, peripheral neuropathy
How to cite this article:
Nwabueze SA, Joe-Ikechebelu NN, Modebe IA, Adogu PO, Ele PU. Relationship between peripheral neuropathy and antiretroviral drugs used in the management of adult human immunodeficiency virus patients. J HIV Hum Reprod 2013;1:36-40 |
How to cite this URL:
Nwabueze SA, Joe-Ikechebelu NN, Modebe IA, Adogu PO, Ele PU. Relationship between peripheral neuropathy and antiretroviral drugs used in the management of adult human immunodeficiency virus patients. J HIV Hum Reprod [serial online] 2013 [cited 2017 Apr 8];1:36-40. Available from: http://www.j-hhr.org/text.asp?2013/1/1/36/116529 |
| Introduction | |  |
Anti-retroviral (ARV) therapy (ART) has led to a revolution in the care of patients with human immunodeficiency virus (HIV) and acquired immune deficiency syndrome (AIDS) in the developed world. Treatment is not a cure but a lifelong therapy. It presents with new challenges of side-effects and drug resistance even though it dramatically reduces the rate of mortality and morbidity, while also improving quality of life to people living with HIV/AIDS. [1] Adverse reaction related to the use of ARV drugs may severely jeopardize patients' confidence in the safety of these medicines and alter their adherence to ART. This will not only lead to possible reduction in the treatment efficacy of the ARVs with increased morbidity and mortality but will also negatively affect treatment program's effectiveness and increase the risk of emergence of secondary drug resistance. New adverse events and toxicities are identified as people live longer on ART. For these reasons, pharmaco-vigilance has become an integral part of ARV program. Sadly, the reporting of adverse drug reaction (pharmaco-vigilance) for all the medicine has been poor, and remains limited in the case of ARV agents. [1]
A variety of peripheral neuropathies (PN) are associated with HIV infection. [2] Although the incidence of certain forms of neuropathy is increased in HIV infection, in other cases, the association may be fortuitous. Different forms of peripheral neuropathy occur with increased frequency at particular stages of HIV disease. The incidence of distal symmetrical poly-neuropathy (DSP), for instance, increases as immune-suppression progresses and HIV viral load becomes uncontrolled. [2] However, a number of symmetrical forms of DSP occur in association with HIV infection and ARV therapy. Although Zidovudine (AZT) is limited by hematologic toxicity, there is no evidence that AZT causes DSP. [3] The dideoxynucleoside analogs Didanosine (ddI), Zalcitabine (ddC), and Stavudine (d4T) have well-recognized neurotoxicity. [4] Unexpected peripheral neuropathy was first described in patients receiving ddC. [5] The acute onset and rapid progression of nucleoside-related PN, and particularly the beneficial effect of drug withdrawal, serve to distinguish HIV-associated PN from nucleoside toxicity. [5],[6] In general, the choice of regimen depends on their side-effects, potential drug interactions, co-morbidities (e.g., tuberculosis [TB]), alternative options in the setting of treatment failure, drug availability, and cost. [7] Several other drugs used in the treatment of HIV-related complications may cause PN. [3] For instance, majority of patients with lymphoma or Kaposi's sarcoma that are treated with chemotherapeutic regimens, particularly vincristine, develop symptoms and signs of PN. Also, peripheral neuropathy may develop in patients treated with isoniazid (INH) for TB, particularly when pyridoxine is not given. [3]
This study seeks to explore the association between PN and ARVs used in management of adult HIV patients accessing care in NAUTH Nnewi.
| Methodology | |  |
Setting
The Nnamdi Azikiwe University Teaching Hospital (NAUTH), Nnewi, is a federal tertiary health institution in South-Eastern Nigeria. The staff strength is 1714, of which 988 are professional personnel, including over 70 consultants in diverse fields of medicine. [8] NAUTH Nnewi provides a wide range of medical, surgical, diagnostic, outpatient, inpatient, rehabilitative, and support services to a catchment population of about 16,395,555. [9] The catchment area spans all through Anambra, Imo, Abia, Delta, Enugu, Ebonyi, and Kogi States. HIV services available in NAUTH include HIV counseling and testing, adherence counseling, ARV therapy, treatment of opportunistic infections, prevention of mother to child transmission (PMTCT), and support groups for patients and home-based care. The HIV clinic runs from Monday to Friday from 8 am to 4 pm every day. Over 12,000 people living with HIV and AIDS People Living With HIV/AIDS (PLWHAs) are enrolled for care. [8]
| Materials and Methods | |  |
This study is a review of medical records of patients accessing HIV care at NAUTH over a 4-year period. The NAUTH electronic adverse events data base, which is a register of adult HIV patients accessing care in the hospital, was searched and data of all adult enrollees in the NAUTH HIV and AIDS program accessing care between July 2005 and July 2009 were reviewed. The inclusion criteria were adult HIV patients without PN at commencement of treatment with ARVs and any other drugs used in treatment of the patient. Conversely, pediatric patients accessing HIV care in NAUTH, adult HIV patients with central nervous system (CNS) complications, and patients with PN at baseline and diabetes mellitus (DM) were excluded from the study.
All the eligible PN patients identified during the review period were recruited into the study. Data extracted from patients' medical records included age, gender, marital status, highest educational level attained, time of onset of symptoms, presence of co-morbidities like TB, hypertension, and malignancies if any; use of ARV drugs like ddI, ddC, d4T, AZT, Tenofovir; offending drugs; laboratory results including CD4 counts at diagnosis of PN; any other drugs used in treatment of the subjects; substituted drug regimens and treatment outcome categorized as resolution or persistence following the drug's substitution.
SPSS version 17 statistical software was used to analyze the data, while the Chi-squared test of significance was conducted with accepted level of statistical significance set at P value <0.05. In this way, the factors associated with prevalence of PN as well as PN outcomes were examined.
Permission for this study was obtained from the NAUTH Ethics Committee. The limitation of this study was that detailed neurologic evaluation was not performed in most patients and this makes the application of the WHO clinical classification method difficult.
| Results | |  |
The highest incidence of peripheral neuropathy was recorded among the widowed female subjects and those aged >40 years (P < 0.05 and P < 0.01, respectively) [Table 1]. Median duration of HIV treatment with ARV was 42 weeks.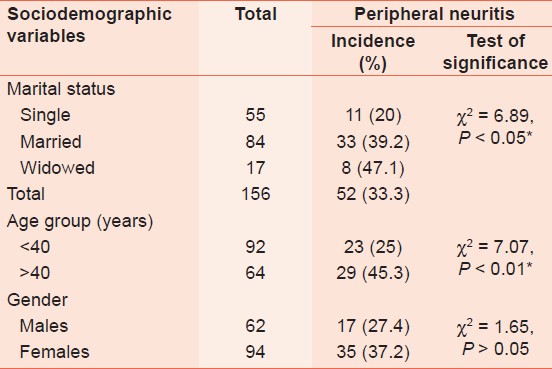 | Table 1: Peripheral neuropathies incidence and sociodemographic variables
Click here to view |
The relationship between socio-demographic variables and PN outcome is shown in [Table 2]. Females and patients >40 year age group have statistically significant higher proportion of resolved PN than their male and <40 year-old counterparts (P < 0.05 and P < 0.01, respectively).
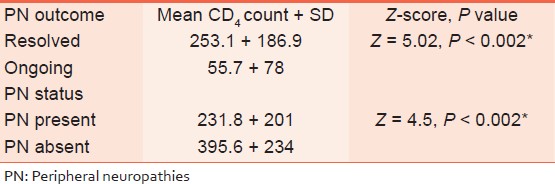
Furthermore, the mean CD 4 count of subjects who had resolved PN was significantly higher than that of those whose PN was ongoing (P < 0.002). Similarly, the subjects who had no PN recorded a significantly higher CD 4 count than those found to have PN (P < 0.002) [these are shown in [Table 3].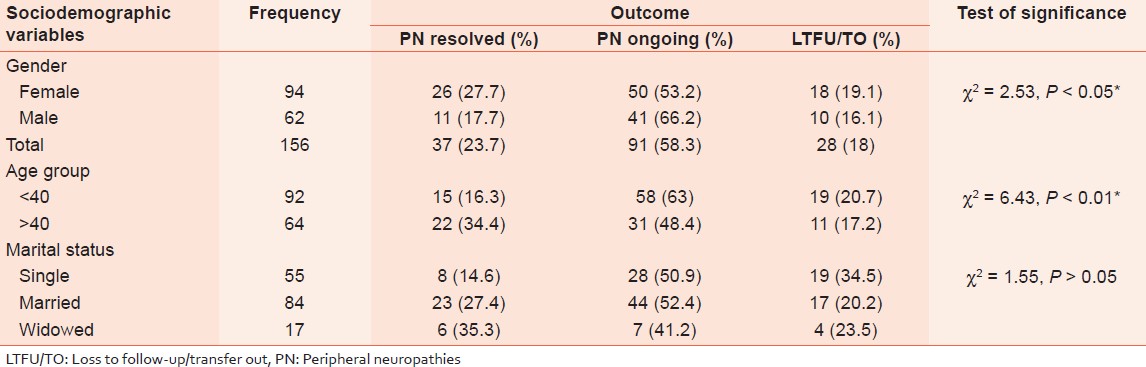 | Table 2: Peripheral neuropathies outcome and sociodemographic variables
Click here to view |
 | Table 3: Relationship of CD4 count with peripheral neuropathies outcome/status
Click here to view |
A higher proportion of cases of PN were found among subjects on offending drugs (the drugs that have PN as complication or side-effect) than among those on alternate drugs [Table 4]. This is significant at P < 0.005.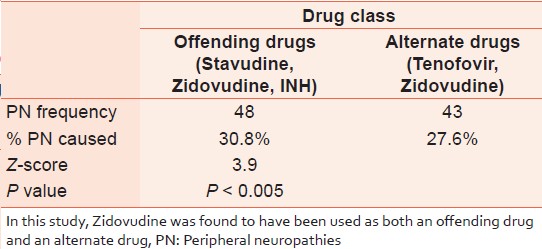 | Table 4: Peripheral neuropathies among subjects on offending versus alternate drugs
Click here to view |
Hypertension (2.4%) and TB (3.7%) were the most prevalent co-morbidities. Other co-morbidities, psychosis and lymphoma, were each found in 0.6% of the subjects. Also, while 100% of subjects with co-morbidity presented with PN, only 27.8% of respondents without co-morbidity had PN (P < 0.001).
| Discussion | |  |
This study clearly indicates a statistically significantly higher proportion of patients (33.3%) developing PN on taking offending drugs [d4T and AZT (which is minimally offending)] compared with the proportion who took the alternative drugs (Tenofovir and AZT). This is similar to result of the study conducted in Uganda in 2009 in which a total of 860 adult ARV-naïve participants were initiated on d4T-containing treatment regimen, and 308 (35.8%) of these reported symptoms of peripheral neuropathy within median of 48 days after ARV drug initiation. [10] A higher proportion (56%) of patients in a Malawian cohort developed peripheral neuropathy. [11] It is, however, at variance with result of the study in 2008 where only 10-21% of persons exposed to d4T reported peripheral neuropathy in developed countries. [12] This discrepancy between developing and developed countries PN prevalence may have arisen because of differences in environmental conditions and nutritional status prevalent in both worlds. Related to this are the possible differences in co-morbidity rates among the subjects in the two worlds.
It is remarkable that in this study, the patients recorded a median duration of 42 weeks of treatment with d4T before developing PN. Similar results have been obtained in other studies in which persons exposed to d4T developed peripheral neuropathy within 28 weeks, and in rural Uganda in which 9% of a cohort of 1029 persons under d4T/Lamivudine/Nevirapine or Efavirenz developed severe-graded PN after a median follow-up of 45 weeks. [12] Also, in Kenya, in a prospective cohort of 1286 patients under d4T, neuropathy was the highest reported toxicity (20.7% of patients) after a median follow-up time of 47 weeks. [13] Optimum time to substitute with a non-d4T-containing regimen is unknown, and further studies are required to determine this appropriate substitution time with a view to ensuring an improved quality of life for the patients. Symptoms generally resolve with prompt discontinuation of d4T, but may persist in a subset of patients. [13] Persistent symptoms in such patients may be problematic in developing countries, where many persons rely on physical labor for survival and usually do not have disability insurance. [11]
Age has significantly influenced PN incidence in this study. There are significantly greater proportions of PN among patients aged >40 years than among their counterparts who are aged 40 years or below. This is hardly surprising especially when it is known that gradual changes to the peripheral nervous system, including the potential decline of peripheral nerve function, are associated with the normal aging process. [14] Furthermore, the incidence of PN was higher in females even though there were more resolved cases among them than among the males. This finding is similar to that in another study published in the journal of clinical pharmacology, which found a statistically significant correlation between female gender and severe ADR. It is not clearly known why gender difference exists in adverse reactions to ARV drugs. However, factors cited include differences in weight and body mass index, hormonal changes unique to females, and the effect of these changes on drug metabolism. [15] Other possible factors include differences in fat composition (thereby affecting drug distribution) and genomic differences influencing the level of enzymes involved in drug metabolism. [16]
Both resolved PN and absence of PN were associated with significantly higher mean CD 4 count than was found in ongoing and presence of PN, respectively. ADR to ART may depend on the baseline CD 4 cell count at initiation of therapy. A study by Center for Disease Control and Prevention on HIV outpatients suggested that some complications were more frequent and severe when therapy was started at lower CD 4 cell counts. Thus, patients may actually experience fewer side-effects over a 10- to 20-year period of drug exposure if they start therapy 18-24 months earlier than if they delay therapy until the CD 4 cell count decreases to less than 0.200 × 10 9 cells/L. [17]
TB and hypertension were recorded as the most prevalent co-morbidities among the subjects in this study. All subjects with co-morbidities were also found to present with PN suggesting a possible link between the two variables. This is expected especially in TB the treatment of which involves the use of a potentially PN-causing INH, more so when a Malawian cohort study demonstrated an associated neuropathy in TB treatment despite using pyridoxine with INH. [11] It is also similar to the findings in another study on HIV neuropathy among South Africans, which found that PN was independently associated with ART use and prior TB. [17]
In conclusion, despite the adverse reactions of PN, ART is the only available treatment of choice for HIV and AIDS. The management of HIV with ARVs requires a balance between benefits of durable HIV suppression and the risks of drug toxicity to achieve the therapeutic goals in the absence of less toxic agents. Patient adherence can be improved with proper education and counseling regarding the disease process and inherent but innocuous side-effects of highly active anti-retroviral therapy (HAART). More research is needed to develop algorithms for prediction of adverse effects of existing regimen, along with evolution of more efficacious and less toxic medicines.
| References | |  |
| 1. | World Health Organization. Cancer pain relief and palliative care. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser 1990;804:1-75. 
|
| 2. | So YT, Holtzman DM, Abrams DI, Olney RK. Peripheral neuropathy associated with acquired immunodeficiency syndrome. Prevalence and clinical features from a population-based survey. Arch Neurol 1988;45:945-8. 
[PUBMED] |
| 3. | Simpson D, Olney R. Peripheral neuropathies associated with human immunodeficiency virus infection. In: Duck PJ, editor. Peripheral Neuropathies: New Concepts and Treatments, Neurologic Clinics. Philadelphia, PA: WB Saunders; 1992:685-711. 
|
| 4. | Simpson DM, Tagliati M. Nucleoside analogue-associated peripheral neuropathy in human immunodeficiency virus infection. J Acquir Immune Defic Syndr Hum Retrovirol 1995;9:153-61. 
[PUBMED] |
| 5. | Dubinsky RM, Yarchoan R, Dalakas M, Broder S. Reversible axonal neuropathy from the treatment of AIDS and related disorders with 2´,3´-dideoxycytidine (ddC). Muscle Nerve 1989;12:856-60. 
[PUBMED] |
| 6. | Berger AR, Arezzo JC, Schaumburg HH, Skowron G, Merigan T, Bozzette S, et al. 2´,3´-dideoxycytidine (ddC) toxic neuropathy: A study of 52 patients. Neurology 1993;43:358-62. 
|
| 7. | World Health Organization. Scaling up antiretroviral therapy in resource limited settings guidelines for a public health approach; 2002. Available from: http://www.who.int/hiv/pub/prev_care/en/ScalingUp_E.pdf. [Last accessed on 2012 Apr]. 
|
| 8. | Modebe IA, Adinma ED, Nwabueze SA. 5-year strategic plan 2007-2011. Nnewi: Nnamdi Azikiwe University Teaching Hospital; 2006. 
|
| 9. | Federal Republic of Nigeria Official Gazette Abuja. 2006 Nigerian national census. 2009;96:B21-34. 
|
| 10. | Gerald M, Moore D, Were W, Malamba S, Mermin J, Weidle P, et al. Outcome of stavudine-induced peripheral neuropathy in HIV-infected individuals in rural Uganda. Conf Retrovir Opportunistic Infect 2008;15:442. 
|
| 11. | Beadles WI, Jahn A, Weigel R, Clutterbuck D. Peripheral neuropathy in HIV-positive patients at an antiretroviral clinic in Lilongwe, Malawi. Trop Doct 2009;39:78-80. 
[PUBMED] |
| 12. | Breen RA, Lipman MC, Johnson MA. Increased incidence of peripheral neuropathy with co-administration of stavudine and isoniazid in HIV-infected individuals. AIDS 2000;14:615. 
[PUBMED] |
| 13. | Makinson A, Moing VL, Kouanfack C, Laurent C, Delaporte E. Safety of stavudine in the treatment of HIV infection with a special focus on resource-limited settings. Expert Opin Drug Saf 2008;7:283 93. 
[PUBMED] |
| 14. | McCarthy KA. Peripheral neuropathy in the aging patient: Common causes, assessment, and risks. Top Clin Chiropr 1999;6:56-61. 
|
| 15. | Tran C, Knowles SR, Liu BA, Shear NH. Gender differences in adverse drug reactions. J Clin Pharmacol 1998;38:1003-9. 
[PUBMED] |
| 16. | Harris RZ, Benet LZ, Schwartz JB. Gender effects in pharmacokinetics and pharmacodynamics. Drugs 1995;50:222-39. 
[PUBMED] |
| 17. | Maritz J, Benatar M, Dave JA, Harrison TB, Badri M, Levitt NS, et al. HIV neuropathy in South Africans: Frequency, characteristics, and risk factors. Muscle Nerve 2010;41:599-606. 
[PUBMED] |
[Table 1], [Table 2], [Table 3], [Table 4]
|