|
 
 |
| ORIGINAL ARTICLE |
|
| Year : 2015 | Volume
: 3
| Issue : 1 | Page : 7-13 |
|
Effectiveness of prevention of mother-to-child transmission of HIV program in Abuja, Nigeria
Chris O Agboghoroma1, Lamidi Isah Audu2, Kenneth C Iregbu3
1 Department of Obstetrics and Gynaecology, National Hospital, Abuja, Nigeria
2 Department of Paediatrics, National Hospital, Abuja, Nigeria
3 Department of Medical Microbiology and Parasitology, National Hospital, Abuja, Nigeria
| Date of Web Publication | 9-Nov-2015 |
Correspondence Address:
Chris O Agboghoroma
Department of Obstetrics and Gynaecology, National Hospital, Abuja
Nigeria
 Source of Support: None, Conflict of Interest: None  | Check |
DOI: 10.4103/2321-9157.169176

Aim: The aim of this study is to evaluate the effectiveness of prevention of mother-to-child transmission (PMTCT) of HIV program in a tertiary level health facility in Abuja, Nigeria. Materials and Methods: It was a retrospective study. Records of HIV positive pregnant women who registered and had antenatal care (ANC) in the period January 1, 2006 to December 31, 2008, delivered in the hospital and whose HIV-exposed babies were followed-up to 6 weeks of age when HIV status was determined by DNA polymerase chain reaction techniques were collected and analyzed. Results: During the 3 years period, 643 pregnant women were HIV-positive. Among these group, 495 delivered in the hospital and 247 had their babies followed-up till the point of HIV testing. The overall MTCT rate was 2.4%, mothers who used triple combination antiretroviral (ARV) therapy or prophylaxis recorded MTCT rate of 1.3% while mothers who received only intrapartum single dose nevirapine had MTCT rate of 37.5%. The majority of the mothers, 186 (76.5%) were delivered by caesarean section, and the MTCT rate in this group was 1.6% while the MTCT rate in mothers who delivered vaginally was 5.5%. Exclusive formula feeding was practiced by 232 (96%) mothers, and had a MTCT rate of 2.1%. While the MTCT rate in mothers who practiced exclusive breastfeeding was 12.5%. Conclusions: The MTCT rate in the PMTCT program in this study compared favorably with reports from other centers and developed countries where similar interventions are the standard care for HIV-positive women. The finding demonstrates that routine provision of comprehensive PMTCT services including triple combination ARV drugs is feasible and effective in our setting.
Keywords: Antiretroviral drugs, césarien section, exclusive breastfeeding, exclusive formula feeding, HIV, mother-to-child transmission, prevention
How to cite this article:
Agboghoroma CO, Audu LI, Iregbu KC. Effectiveness of prevention of mother-to-child transmission of HIV program in Abuja, Nigeria. J HIV Hum Reprod 2015;3:7-13 |
How to cite this URL:
Agboghoroma CO, Audu LI, Iregbu KC. Effectiveness of prevention of mother-to-child transmission of HIV program in Abuja, Nigeria. J HIV Hum Reprod [serial online] 2015 [cited 2018 Aug 6];3:7-13. Available from: http://www.j-hhr.org/text.asp?2015/3/1/7/169176 |
| Introduction | |  |
HIV infection is an important cause of morbidity and mortality in children in Africa.[1] Over 90% of HIV infections in children are acquired through the mother-to-child transmission (MTCT) route. About 25–40% of HIV-positive women will transmit the virus to their children during the period of pregnancy, labor/delivery, and breastfeeding if there is no intervention.[2] With appropriate interventions which include use of antiretroviral (ARV) drugs, obstetric interventions and modification of infant feeding, MTCT rates have been reduced to <2% in some countries.[3],[4],[5] This has significantly reduced the incidence of pediatric HIV/AIDS and associated morbidity and mortality in those countries.
In Nigeria, the sentinel surveys of pregnant women attending antenatal clinic revealed persistently high HIV prevalence of 5.8%, 5%, 4.4%, 4.6%, and 4.1% in 2001, 2003, 2005, 2008 and 2010 respectively.[6],[7],[8],[9],[10] The high prevalence of HIV in the country, coupled with a high total fertility rate of 5.5 births per woman, crude birth rate of 41/1000 population and the cultural practice of prolonged breastfeeding often with mixed feeding (MF) make vertical transmission an important route of spread of HIV in Nigeria.[11] It is estimated that about 69,000 infants contract HIV infections from their mothers annually in Nigeria during pregnancy, delivery, and breastfeeding periods.[12] As part of efforts to halt this trend, the Federal Government of Nigeria with support from development partners initiated the prevention of MTCT (PMTCT) of HIV program in 2001. The National PMTCT program was initially based in National Hospital Abuja and five other sites in the six geopolitical zones of the country. This has been scaled-up to involve other tertiary, secondary and some primary health care centers.[13],[14]
Many studies from developing countries that demonstrated potentials for a reduction in MTCT of HIV with the use of ARV drugs were conducted mainly in research centers utilizing less potent short-course ARV therapy with single or dual combination drugs.[15],[16],[17],[18] This study evaluated the effectiveness of the PMTCT interventions at the National Hospital Abuja, Nigeria where triple combination ARV drugs – highly active antiretroviral therapy (HAART) were used for PMTCT interventions under routine clinical setting.
| Materials and Methods | |  |
Study design
This was a retrospective study nested within a routine PMTCT program at a tertiary level health facility located in the metropolis of the Federal Capital Territory, Abuja, Nigeria. The Department of Obstetrics and Gynecology provides a comprehensive range of reproductive health care services which include ANC, delivery care, postnatal care, gynecological care, family planning and screening for cervical cancer. MTCT interventions are implemented in accordance with standard PMTCT practice and standard operating procedures.[19],[20] It essentially involves HIV counseling and testing of pregnant women who presented for ANC and the provision of interventions for HIV-positive women to reduce the risk of transmitting the infection to the newborn. The interventions offered during the period under review include clinical and immunological assessment, triple combination ARV drugs – HAART for treatment or prophylaxis based on 200 cells/mm 3 CD4 cut-off point, professional antenatal and safe delivery care including caesarean section (CS), counseling and care related to infant feeding and psychological support. The HIV-exposed babies also received ARV prophylaxis, expert follow-up care and early infant diagnosis (EID) for HIV. The PMTCT program at the National Hospital Abuja receives technical, laboratory, ARV drug and infant formula support from government institutions and development partners.
Between 2001 and 2004, single-dose maternal intrapartum nevirapine (NVP) and single dose neonatal NVP (within 36 h of delivery) were the only ARV drugs used in the PMTCT program and the exposed babies HIV status were determined only after 18 months.[21] Subsequent review of the National PMTCT guidelines in 2005 prescribed options of ARV drugs including triple combination therapy – HAART for the mother, while the infants received single-dose NVP (Sd-NVP) syrup 2 mg/kg and zidovudine (ZDV) syrup 4 mg/kg for 6 weeks, irrespective of the maternal drug regime.[22] Early infant diagnosis (EID) at 6 weeks using DNA polymerase chain reaction (PCR) technology also became available in the facility in 2005.
Study population
The study covers HIV positive pregnant women who registered and received ANC between January 1st 2006 and December 31st 2008, delivered in the National Hospital, Abuja and infants followed-up for 6 weeks and beyond until HIV status was determined by DNA PCR technology. During the study period, HIV-positive women who were on antiretroviral treatment (ART) – HAART prior to becoming pregnant were continued on their medication throughout the period of pregnancy, delivery and postpartum. The use of efavirenz (EFV) was avoided in the first trimester, and ZDV inclusion in the drug regimen was considered essential. Women diagnosed HIV-positive for the first time during pregnancy were assessed for their eligibility for ARV therapy for their health. Those that qualified for treatment for their health (CD4 count ≤200 cells/mm 3) were placed on ART – HAART throughout the period of pregnancy, delivery, and postpartum. Those with CD4 count >200 cells/mm 3 were placed on ARV prophylaxis – HAART during pregnancy and delivery and subsequently followed-up in the special treatment clinic. The triple drug combination used as HAART includes ZDV 200 mg BD, lamivudine (3TC) 150 mg BD and NVP 200 mg BD. If the CD4 count was > 250 cells/mm 3 NVP may be substituted with EFV or a protease inhibitor such as indinavir, saquinavir or lopinavir/ritonavir. In a situation where the client had not commenced ARV drugs before the onset of labor, maternal Sd-NVP 200 mg was given in labor followed with ZDV and 3TC for 7 days in the postpartum period. The HIV-exposed infants were followed-up in the pediatric clinic for infant feeding support, growth monitoring, immunization, ARV prophylaxis and EID. Sd-NVP syrup 2 mg/kg and ZDV syrup 4 mg/kg for 6 weeks were given to the exposed infants, irrespective of the maternal drug regimen. The prevailing government policy during the period under study made PMTCT services including ANC, delivery (both caesarean and vaginal) and ARV drugs free in federal medical facilities in the country. Infant formula was supplied free to mothers who opted for formula feeding. The mode of delivery and infant feeding methods were based on clients'choice following counseling.
The primary endpoint for the study was infant HIV infection status determined after 6 weeks of birth. All infants diagnosed HIV-positive were enrolled into the care and treatment program and initiated on ARV therapy according to the national guidelines.
Laboratory procedures
Maternal serostatus was determined using the parallel screening algorithm tests that utilize two rapid tests – Capillus ® test kit (Trinity–USA) and Genie ® test kit (BioRad–France) to identify anti-HIV-1 and anti-HIV-2 antibodies. Concordance positive test were confirmed positive. Discordant result for the above two kits were resolved using a third test kit–tie breaker, e.g. Determine ® (Abbot-Japan). The EID were performed from dried blood spot samples collected from exposed infants at/after 6 weeks of age and processed using DNA PCR technology. Those that were positive were confirmed with a second infant specimen. Mothers who were breastfeeding had their infants tested again, 6 weeks after the cessation of breastfeeding. Maternal viral load assessment was not available in the facility during the study period.
Data collection and statistical analysis
Mother-infant pairs data were extracted from medical files and PMTCT registers and entered into questionnaires designed for the study. The variables collected included maternal age, employment status, parity, and gestational age at booking, gestational age at delivery, time of HIV diagnosis, type of ARV regimen, time of commencement, CD4 cell count, mode of delivery, infant feeding practice and infant HIV status. Data were entered and processed using Epi-Info ™ version 3.5.1 statistical software (USD Incorporated, Georgia).[23] Descriptive data analysis was done using the same software. Transmission rates were determined for the various interventions.
| Results | |  |
Between January 2006 and December 2008, there were 5539 pregnant women who booked for ANC and were tested for HIV. Six hundred and forty-three of the women were HIV positive, giving an HIV seroprevalence of 12% among the pregnant women. Four hundred and ninety-five (77%) of the HIV-positive mothers delivered in the hospital. The study is based on the 247 mother-infant pairs that were followed-up from the point of antenatal booking through birth till 6 weeks and beyond when the infants HIV status were determined by DNA PCR testing. The yearly mother-infant pair's statistics is summarized in [Table 1].
The median age of the 247 mothers was 30 years (interquartile range [IQR] 27–33 years). One hundred and sixteen (47%) were unemployed. One hundred and forty-four (58%) were experiencing their first pregnancy or had only one previous delivery. More than 77% (190/247) were aware of their HIV status before the index pregnancy. The median CD4 count was 360 cells/mm 3 (IQR 250–496 cells/mm 3). The median gestational age at first antenatal attendance was 19 weeks (IQR 14–25 weeks). Over 94% of the mothers were on triple combination ART or prophylaxis. Seventy-five percent of the mothers were delivered either through elective or emergency CSs. More than 95% infants had exclusive formula feeding (EFF).
[Table 2] shows the infant HIV status in relation to maternal ARV drug used, mode of delivery and infant feeding method. The overall MTCT rate was 2.4%; there was, however, a gradual reduction in the rate from 4.7% in 2006 to 1% in 2008. The MTCT rate was 1.3% in women who used triple combination ARV drugs compared to 37.5% in women who used Sd-NVP in labor. No MTCT was noted in mothers who commenced ARV drugs in the first and second trimester, MTCT rate of 6.5% occurred in women who commenced ARV drugs in the third trimester. Women who were delivered by CS had lowered MTCT compared with those who had a vaginal delivery. Elective CS was however associated with lower MTCT transmission rates compared with emergency CS. Infants that had EFF had a lowered MTCT rate compared with those who had exclusive breastfeeding (EBF). The infection rates in male infants (2.4%) were similar to that of female infants (2.5%). There was a progressive increase in the proportion of babies followed-up and tested at 6 months from 40% in 2006 to 50% and 55% in 2007 and 2008 respectively.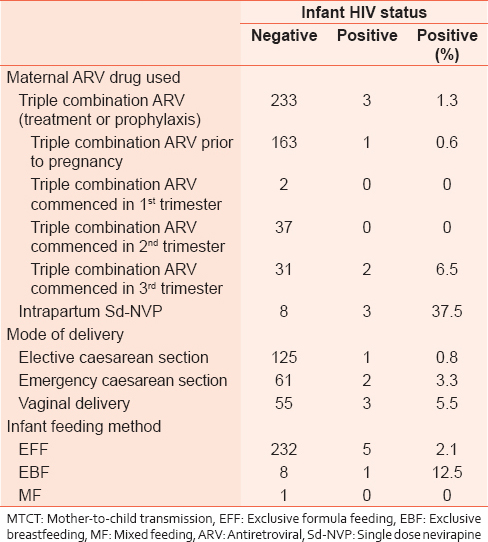 | Table 2: Maternal ARV use, mode of delivery, infant feeding practice and MTCT of HIV
Click here to view |
| Discussion | |  |
This was a retrospective analysis of data from a routine PMTCT program in a tertiary hospital in Nigeria. The study demonstrates that the use of combination ARV drug in PMTCT program in the resource-limited environment is feasible and associated with reduced MTCT rates comparable with those reported from centers in developed countries. While the overall MTCT rate was 2.4%, the MTCT rate was 1.3% in mothers who used triple combination ARV for treatment and prophylaxis. The MTCT rate for mothers who received only Sd-NVP at the time of delivery was 37.5%. The recorded transmission rates were much lower in mothers who were on ARV drugs for longer periods before delivery. Mothers who were on ARV therapy prior to conception and those that commenced ARV in the first and second trimesters had MTCT rate of 0.6% compared to 6.5% in mothers who commenced ARVs in the third trimester. These findings were similar to those reported in other studies.[24],[25],[26],[27]
Although the use of CS delivery has been associated with lower MTCT rates in many research settings.[28],[29] the availability and use of effective triple combination ARV for treatment and prophylaxis which has effectively reduced the maternal viral load to less than 1000 copies/ml or undetectable levels has reduced the need for CS deliveries for PMTCT.[30] The increase in CS rates associated with the effort to reduce MTCT of HIV has been reported in the USA.[31] In our series over 75% of mothers were delivered either by elective or emergency CS. The high CS rate could partly be attributed to the implementation of federal government policy of free antenatal and delivery care (including operative deliveries) for HIV-positive mothers. During the period under review, caesarean delivery was undertaken as a measure for PMTCT in mothers who opted for this mode of delivery after counseling. The inability to undertake viral load study and hence determination of the effectiveness of the ARV therapy also contributed to the high CS rate.
The protective effect of CS was demonstrated in our study. While the MTCT rate for mothers delivered through CS was 1.6% (0.8% and 3.3% respectively for elective and emergency CS), the transmission rate in mothers delivered vaginally was 5.5%. The availability of the facility for maternal viral load assessments will reduce the need for CS in clients who are found to have very low or undetectable viral load at term. However, in settings where women booked late for ANC services, good ART adherence cannot be ensured, and the effectiveness of ART in reducing viral load unknown, CS remains an option of management in the context of PMTCT.
The pattern of infant feeding amongst the mothers showed that majority (over 95%) used EFF. This could be partly attributed to the prevailing policy of provision of free infant formula to HIV-exposed babies during the period under review. Although this study did not include information on infant morbidity and mortality, the use of infant formula has been associated with increased infant morbidity and mortality in developing countries and therefore not recommended for routine use for PMTCT.[32],[33],[34] The potential for postnatal transmission through breast milk was obvious in this study as one infant among the 8 (12.5%) that had EBF was HIV infected. The predominant use of EFF in this study was possibly responsible for the low number of postnatal HIV transmission recorded. Breast milk accounts for up to a third of all MTCT in mothers who breastfeeds their infants without ART or prophylaxis for the mother or the baby. The elimination of postnatal transmission of HIV remains a major challenge in PMTCT program in Nigeria and other African countries where breastfeeding is virtually universal. A recent report showed that 97% of Nigerian women breastfeed their babies, but only 7% practice EBF while the majority practice MF.[11] There is the possibility that many of the mothers who claimed EBF actually did MF. MF has been associated with higher risk of HIV transmission compared with EBF.[33] There is the need to determine and promote safer means of infant feeding in the context of maternal HIV infection and MTCT. This should include the promotion of EBF and avoidance of MF, and ways of making formula feeding acceptable, feasible, affordable, safe, and sustainable in our setting. The extended use of ARV drugs during the period of breastfeeding by the mother and/or the infant has been associated with reduced transmission and currently recommended in situ ations where breastfeeding is commonly practiced.[35],[36],[37] There is the need to determine the effectiveness and safety of this practice in our setting.
While the quality of care for HIV-positive clients in this facility may be considered satisfactory, assessment of mother's HIV-1 viral load was not routinely undertaken during the period studied. It was therefore not possible to determine the effectiveness of the ARV drugs through patient's virologic response. Another limitation in this study was the high loss to follow-up (LTFU) in the antenatal and postnatal periods. Only 77% of mothers who were diagnosed HIV-positive in the antenatal period delivered in the hospital. Of the women who delivered in the hospital, only 50% of mother-infant pairs were seen to the point when EID was made. It is possible that some infected infants were LTFU, and the effectiveness of our PMTCT program was therefore overestimated. The gradual increase in the follow-up rate over the 3 years period may suggest an increasing level of awareness among the mothers. Namukwaya et al.[17] in their study from Uganda also reported a high LTFU of over 50%. Strategies including use of peer support, mentor mothers and mobile phone reminders would need to be adopted to improve antenatal, postnatal and infant follow-up.[38] Despite the limitations, the effectiveness of the PMTCT program, as measured by the MTCT rate in this series, is consistent with reports from other established centers where PMTCT services are rendered.
| Conclusion | |  |
This study showed that provision of comprehensive PMTCT services including triple combination ARV drugs is feasible and resulted in low MTCT rates under routine clinic conditions in resource-limited setting. Commencement of ARV drugs prior to conception or in the first and second trimesters of pregnancy should be emphasized in PMTCT programs.
Although the skills of health personnel, infrastructures, and operational context vary considerably across the various levels of health care in the country, a well-organized system will enable the implementation of PMTCT services in most health care facilities. A well-coordinated 'hub and spoke' arrangement can ensure lower level health care facilities benefit from supervisory and technical support from well-established tertiary and secondary level facilities. Strategies to improve maternal and infant follow-up is desirable for successful PMTCT services in our setting.
| Acknowledgments | |  |
We wish to acknowledge the technical support and resources provided to the PMTCT program in the National Hospital Abuja by the Institute of Human Virology-Nigeria, Federal Ministry of Health, National Agency for the Control of AIDS, Federal Capital Territory Agency for Control of AIDS and UNICEF. We appreciate the cooperation of the women who participated in the PMTCT program. We are grateful to our medical colleagues and other health workers who were involved in the care of HIV-positive women in the National Hospital, Abuja.
| References | |  |
| 1. | Tindyebwa D, Kayita J, Musoke P, Eley B, Nduati R. Handbook on Paediatric AIDS in Africa. Kampala: ANECCA; 2006.  |
| 2. | Rates of mother-to-child transmission of HIV-1 in Africa, America, and Europe: Results from 13 perinatal studies. The Working Group on Mother-To-Child Transmission of HIV. J Acquir Immune Defic Syndr Hum Retrovirol 1995;8:506-10.  [ PUBMED] |
| 3. | Townsend C, Cortina-Borja M, Peckham C, Lyall H, de Ruiter A, Tookey P. Very low risk of mother-to-child transmission of HIV in women on HAART achieving viral suppression in the UK and Ireland. AIDS 2008;22:973-81.  |
| 4. | European Collaborative Study. Mother-to-child transmission of HIV infection in the era of highly active antiretroviral therapy. Clin Infect Dis 2005;40:458-65.  [ PUBMED] |
| 5. | Agboghoroma OC. Prevention of mother-to-child transmission of HIV/AIDS. In: Okonofua FE, editor. Confronting the Challenge of Reproductive Health in Africa: A Textbook for Students and Development Practitioners. Benin: WHARC; 2014. p. 347-68.  |
| 6. | Sani-Gwarzo N, Eloike T, Ekanem EE, Ebong O, Gboun F. A Technical Report on the 2001 National HIV/Syphilis Sentinel Survey among Pregnant Women Attending Antenatal Clinics in Nigeria. Abuja: Federal Ministry of Health; 2001.  |
| 7. | Federal Ministry of Health. Technical Report on the 2003 National HIV/Syphilis Sentinel Survey among Pregnant Women Attending Antenatal Clinics in Nigeria. Abuja: Federal Ministry of Health; 2004.  |
| 8. | Federal Ministry of Health. Technical Report – 2005 National HIV/Syphilis Sero-Prevalence Sentinel Survey among Pregnant Women Attending Antenatal Clinics in Nigeria. Abuja: Federal Ministry of Health; 2006.  |
| 9. | Federal Ministry of Health. Technical Report 2008. National HIV Sero-Prevalence Survey among Pregnant Women Attending Antenatal Clinics in Nigeria. Abuja: Federal Ministry of Health; 2008.  |
| 10. | Federal Ministry of Health (FMOH). Technical Report 2010. National HIV Sero-Prevalence Sentinel Survey among Pregnant Women Attending Antenatal Clinics in Nigeria. Abuja: FMOH; 2010.  |
| 11. | |
| 12. | UNAIDS. Global Report: UNAIDS Report on the Global AIDS Epidemic. Geneva: UNAIDS; 2012.  |
| 13. | National Agency for the Control of AIDS. National HIV/AIDS Response Review 2005-2009. Abuja, Nigeria: National Agency for the Control of AIDS; 2009.  |
| 14. | Agboghoroma OC, Sagay SA, Ikechebelu JI. Nigerian prevention of mother-to-child transmission of human immunodeficiency virus program: The journey so far. J HIV Hum Reprod 2013;1:1-7.   |
| 15. | Wiktor SZ, Ekpini E, Karon JM, Nkengasong J, Maurice C, Severin ST, et al. Short-course oral zidovudine for prevention of mother-to-child transmission of HIV-1 in Abidjan, Côte d'Ivoire: A randomised trial. Lancet 1999;353:781-5.  |
| 16. | Guay LA, Musoke P, Fleming T, Bagenda D, Allen M, Nakabiito C, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet 1999;354:795-802.  |
| 17. | Namukwaya Z, Mudiope P, Kekitiinwa A, Musoke P, Matovu J, Kayma S, et al. The impact of maternal highly active antiretroviral therapy and short-course combination antiretrovirals for prevention of mother-to-child transmission on early infant infection rates at the Mulago national referral hospital in Kampala, Uganda, January 2007 to May 2009. J Acquir Immune Defic Syndr 2011;56:69-75.  |
| 18. | Nyandiko WM, Otieno-Nyunya B, Musick B, Bucher-Yiannoutsos S, Akhaabi P, Lane K, et al. Outcomes of HIV-exposed children in western Kenya: Efficacy of prevention of mother to child transmission in a resource-constrained setting. J Acquir Immune Defic Syndr 2010;54:42-50.  |
| 19. | Agboghoroma OC. Management of HIV in pregnancy: A clinical review. Trop J Obstet Gynaecol 2005;22:65-73.  |
| 20. | Nigerian Federal Ministry of Health. National Standard Operating Procedures for Prevention of Mother-to-Child Transmission of HIV (PMTCT). Abuja: Federal Ministry of Health; 2007.  |
| 21. | Federal Ministry of Health Nigeria. National Guidelines for Prevention of Mother-to-Child Transmission of HIV (PMTCT). Abuja: Federal Ministry of Health; 2001.  |
| 22. | Federal Ministry of Health Nigeria. National Guidelines for Prevention of Mother-to-Child Transmission of HIV (PMTCT). 2 nd ed. Abuja: Federal Ministry of Health; 2005.  |
| 23. | Dean AG, Burton AH, Dicker RC. Epi-info version 3.5.1. A word processing, database and statistics program for epidemiology on microcomputers. Stone Mountain, Georgia: USD Incorporated; 2008.  |
| 24. | Tchendjou P, Same-Ekobo C, Nga A, Tejiokem M, Kfutwah A, Nlend AN, et al. Effectiveness of multidrug antiretroviral regimens to prevent mother-to-child transmission of HIV-1 in routine public health services in Cameroon. PLoS One 2010;5:e10411.  |
| 25. | Cooper ER, Charurat M, Mofenson L, Hanson IC, Pitt J, Diaz C, et al. Combination antiretroviral strategies for the treatment of pregnant HIV-1-infected women and prevention of perinatal HIV-1 transmission. J Acquir Immune Defic Syndr 2002;29:484-94.  |
| 26. | Ikechebelu JI, Ugboaja JO, Kalu SO, Ugochukwu EF. The outcome of prevention of mother to child transmission (PMTCT) of HIV infection programme in Nnewi, southeast Nigeria. Niger J Med 2011;20:421-5.  |
| 27. | Kouanda S, Tougri H, Cisse M, Simpore J, Pietra V, Doulougou B, et al. Impact of maternal HAART on the prevention of mother-to-child transmission of HIV: Results of an 18-month follow-up study in Ouagadougou, Burkina Faso. AIDS Care 2010;22:843-50.  |
| 28. | European Mode of Delivery Collaboration. Elective caesarean-section versus vaginal delivery in prevention of vertical HIV-1 transmission: A randomised clinical trial. Lancet 1999;353:1035-9.  [ PUBMED] |
| 29. | The mode of delivery and the risk of vertical transmission of human immunodeficiency virus type 1 – A meta-analysis of 15 prospective cohort studies. The International Perinatal HIV Group. N Engl J Med 1999;340:977-87.  |
| 30. | Ioannidis JP, Abrams EJ, Ammann A, Bulterys M, Goedert JJ, Gray L, et al. Perinatal transmission of human immunodeficiency virus type 1 by pregnant women with RNA virus loads<1000 copies/ml. J Infect Dis 2001;183:539-45.  |
| 31. | Dominguez KL, Lindegren ML, D'Almada PJ, Peters VB, Frederick T, Rakusan TA, et al. Increasing trend of Cesarean deliveries in HIV-infected women in the United States from 1994 to 2000. J Acquir Immune Defic Syndr 2003;33:232-8.  |
| 32. | Andresen E, Rollins NC, Sturm AW, Conana N, Greiner T. Bacterial contamination and over-dilution of commercial infant formula prepared by HIV-infected mothers in a Prevention of Mother-to-Child Transmission (PMTCT) Programme, South Africa. J Trop Pediatr 2007;53:409-14.  |
| 33. | Mbori-Ngacha D, Nduati R, John G, Reilly M, Richardson B, Mwatha A, et al. Morbidity and mortality in breastfed and formula-fed infants of HIV-1-infected women: A randomized clinical trial. JAMA 2001;286:2413-20.  |
| 34. | UNAIDS, UNICEF, WHO. HIV and Infant Feeding: A Policy Statement Developed Collaboratively by UNAIDS, UNICEF and WHO. Geneva, Switzerland: UNAIDS, UNICEF, WHO; 1998.  |
| 35. | Iliff PJ, Piwoz EG, Tavengwa NV, Zunguza CD, Marinda ET, Nathoo KJ, et al. Early exclusive breastfeeding reduces the risk of postnatal HIV-1 transmission and increases HIV-free survival. AIDS 2005;19:699-708.  |
| 36. | Marazzi MC, Nielsen-Saines K, Buonomo E, Scarcella P, Germano P, Majid NA, et al. Increased infant human immunodeficiency virus-type one free survival at one year of age in sub-saharan Africa with maternal use of highly active antiretroviral therapy during breast-feeding. Pediatr Infect Dis J 2009;28:483-7.  |
| 37. | World Health Organization. Rapid advice: Use of antiretroviral drugs for treating pregnant women and preventing HIV infection in infants. Geneva: World Health Organization; 2009.  |
| 38. | Jones SA, Sherman GG, Varga CA. Exploring socio-economic conditions and poor follow-up rates of HIV-exposed infants in Johannesburg, South Africa. AIDS Care 2005;17:466-70.  |
[Table 1], [Table 2]
|