|
 
 |
| ORIGINAL ARTICLE |
|
| Year : 2015 | Volume
: 3
| Issue : 2 | Page : 96-100 |
|
Shear bond strength of orthodontic brackets after antioxidant treatment on previously bleached teeth: An in vitro study
US Krishna Nayak, Aneeshv Katyal
Department of Orthodontics and Dentofacial Orthopaedics, A.B. Shetty Memorial Institute of Dental Sciences, Mangalore, Karnataka, India
| Date of Web Publication | 15-May-2015 |
Correspondence Address:
Aneeshv Katyal
Department of Orthodontics and Dentofacial Orthopaedics, A.B. Shetty Memorial Institute of Dental Sciences, 5th Floor, Deralakatte, Mangalore - 575 018, Karnataka
India
 Source of Support: None, Conflict of Interest: None  | Check |
DOI: 10.4103/2321-3825.146363

Context: Bleaching of the enamel surface reduces shear bond strength. This can be reversed by use of antioxidants prior to bonding of brackets. Aims: The aim of the study was to verify and validate the improvement in bond strength through the application of antioxidant on previously bleached teeth. Materials and Methods: Thirty-six sound maxillary and mandibular premolars extracted for orthodontic purposes were collected and divided into three groups of 12. Specimens in Group 1 were bleached with 35% hydrogen peroxide as a bleaching agent. Specimens in Group 2 were bleached and subsequently treated with 10% antioxidant (sodium ascorbate). Specimens in Group 3 served as a control. All specimens were stored in artificial saliva for 24 h prior to the bonding of orthodontic brackets. After bracket attachment, the samples were stored in distilled water for 1-week and then tested for shear bond strength using an INSTRON testing machine. Results: The data revealed that there was a significant difference between shear bond strengths of the bleached group; bleached + antioxidant group and the control group. Group 2 showed greater bond strength compared with Group 1, but lesser than the bond strength of the control group. Conclusion: The results confirmed the positive effect of antioxidant treatment on the bond strength of orthodontic brackets after tooth bleaching. Keywords: Antioxidants, bleaching, shear bond strength
How to cite this article:
Krishna Nayak U S, Katyal A. Shear bond strength of orthodontic brackets after antioxidant treatment on previously bleached teeth: An in vitro study. J Orthod Res 2015;3:96-100 |
How to cite this URL:
Krishna Nayak U S, Katyal A. Shear bond strength of orthodontic brackets after antioxidant treatment on previously bleached teeth: An in vitro study. J Orthod Res [serial online] 2015 [cited 2018 Sep 6];3:96-100. Available from: http://www.jorthodr.org/text.asp?2015/3/2/96/146363 |
| Introduction | |  |
Stains and discoloration, whether intrinsic or extrinsic, are a result of many factors, which makes them difficult to prevent. [1] Bleaching agents have become popular due to their safe, effective and predictable nature. [2] These agents are effective in whitening teeth suffering from discoloration due to food, tetracycline staining, and fluorosis or mottling. [3] Since the first at-home bleaching system was introduced in 1989, several whitening systems have been developed, which have incorporated peroxide - based compounds of varying concentrations. [4] Patients who are aesthetically oriented increasingly request dental bleaching due to its effectiveness and wide availability. [2],[4] In addition, the number of adults seeking orthodontic treatment is increasing along with an increased likelihood of these patients reporting a previous history of bleaching.
A disadvantage of tooth bleaching prior to orthodontic treatment is a considerable reduction or loss of bond strength between orthodontic brackets and enamel. [5],[6] This is reportedly caused by hydrogen peroxide solutions producing a change in surface morphology, structure and characteristics of enamel. [7],[8] This has alerted clinicians to the possibility that peroxide bleaching might have detrimental effects on enamel and subsequently caused a change in bonding efficiency. Several studies have produced conflicting views, [3],[5],[9],[10] but generally determined that a safe period for bonding may vary between 24 h and 4 weeks following the bleaching procedure. [5],[12],[13]
It has been considered that the pretreatment of bleached teeth with an antioxidant reverses the detrimental effect of the bleaching agent on adhesive bond strength. [11] Therefore, the aim of the present study is to verify and validate the use of an antioxidant prior to bracket bonding in order to enhance the bond strength of bleached teeth.
| Materials and Methods | |  |
This study was conducted in the Department of Orthodontics and Dentofacial Orthopedics, A.B Shetty Memorial Institute of Dental Sciences, Mangalore, Karnataka, India in association with 3M Innovation Center, Electronics City, Bangalore.
Methodology
Thirty-six sound maxillary and mandibular premolars extracted for orthodontic purposes were collected. It was ensured that the buccal enamel was undamaged following extraction, and any residue on the teeth was removed under tap water and using air-water spray. The teeth were stored under refrigeration at 4°C in artificial saliva solution.
Preparation of Specimens
For the purpose of mounting on an INSTRON Testing Machine, the samples were prepared in the following manner. 80 mm × 10 mm × 5 mm brass bars were fabricated and invested in dental plaster to produce a mold. Self-cure acrylic was poured, polymerized and trimmed with cutting disks to a dimension of 40 mm × 10 mm × 5 mm and subsequently finished and polished. Thirty-six bars were fabricated upon, which 36 hollow, open-ended cylinders of 20 mm height and 15 mm internal diameter were cut out of poly (vinyl chloride) (PVC) pipe. Each PVC cylinder was filled with self-cure acrylic into which a premolar tooth was embedded leaving its crown exposed and parallel to a long axis of the cylinder. On the other end of the cylinder, the acrylic bar was inserted parallel to a long axis of the cylinder. In this manner, 36 samples were obtained.
The labial surface of each premolar sample was cleaned using an ultra-sonic scaler and polished with oil-free pumice using a micro brush and stored according to their group in artificial saliva solution.
Experimental Groups
The samples were randomly divided into three groups, each containing 12 samples; Group 1 being the bleached group, Group 2 was the bleached + antioxidant group and Group 3 served as the control group.
Bleaching Procedure
All 12 samples were washed and air-dried. About 35% hydrogen peroxide bleaching gel (Opalescence Xtra [Ultadent Products Inc.]) was applied to the enamel surface of each embedded tooth using an applicator tip following which the gel was light cured according to the manufacturer's instructions.
After completion of bleaching procedure the samples were thoroughly rinsed with a compressed air/water syringe for 30 s, air-dried and stored in artificial saliva solution for 24 h prior to bonding [Figure 1].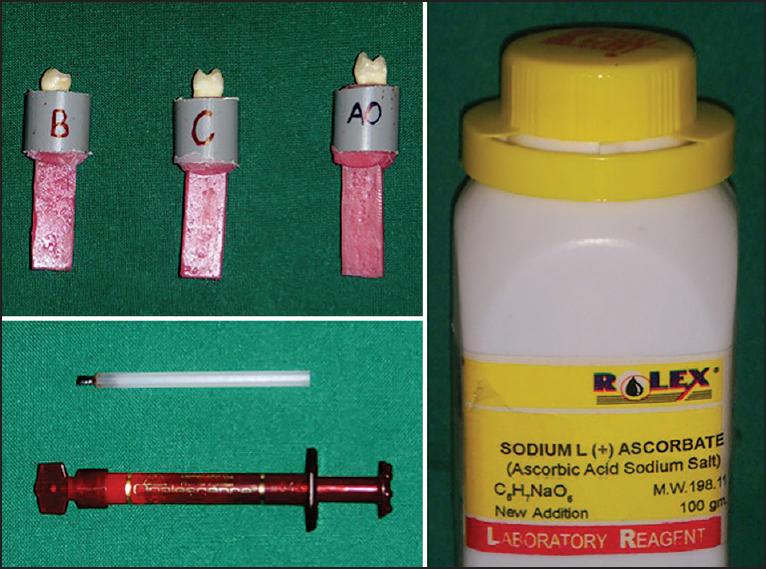 | Figure 1: Mounted specimens; Ultradent Opalascence Xtra whitening agent and 10% sodium ascorbate (antioxidant)
Click here to view |
Application of Antioxidant
All 12 samples of Group 2 were treated in exactly the same way as the samples of Group 1. In addition to bleaching, each sample was treated with an antioxidant solution of 10% sodium ascorbate. A volume of 10 ml of the 10% sodium ascorbate solution was dripped onto the enamel surfaces of the embedded teeth and agitated with a sterile brush. After 10 min, the sample was washed with distilled water and stored for 24 h prior to bonding.
Bonding of Brackets
Thirty-six premolar stainless steel, standard edgewise, twin brackets with a mesh base (ODP - Magnum Roth .022) were used in this study. The brackets were bonded to each sample using a chemically cured composite resin (3M Unitek Transbond). Each sample was washed and air-dried. For all samples, etchant was applied to the buccal surface for 30 s after which the tooth was washed with water and dried with compressed air.
An equal amount of light cure primer (3M Unitek Transbond XT Primer) was applied and cured using a halogen light-curing unit for 15 s. Immediately, after the application of primer, equal amounts of 3M Unitek Transbond XT adhesive paste were applied on each orthodontic bracket base. The brackets were seated and positioned firmly in the middle third of the buccal enamel surface. Excessive resin was removed, and the sample was cured using halogen light (QHL-75, DENTSPLY) for 45 s. After bonding, the samples were stored in distilled water for 1-week before testing for shear bond strength [Figure 2] and [Figure 3].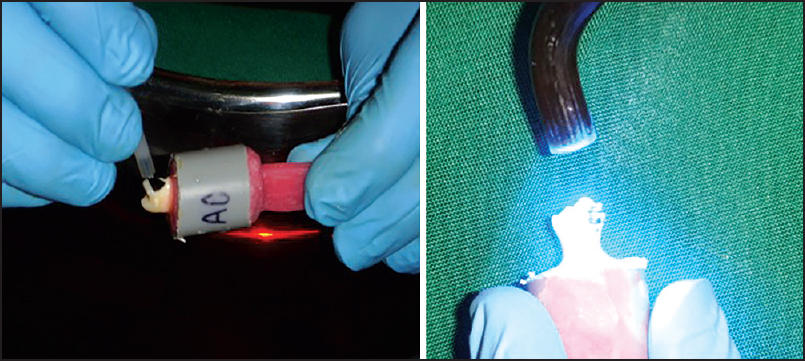 | Figure 3: Application of antioxidizing agent and bonding of orthodontic brackets
Click here to view |
Analysis of Shear Bond Strength
The shear bond strengths of the samples were measured with an INSTRON testing machine after the samples were securely clamped and aligned in an upright position on the lower jaw of the machine. A shearing wire was secured on the upper jaw of the machine and was lowered to engage the orthodontic bracket of the sample held by the lower jaw. The crosshead speed of the INSTRON was 100 N at 1 mm/min. The load at failure was recorded and processed using the Lloyd's DAPMAT Software (version 2.31, Hampshire, UK) [Figure 4].
| Results | |  |
[Table 1] contains the mean, standard deviation and range of the shear bond strength of specimens in each of the three study groups. These descriptive statistics clearly indicate the variation in shear bond strength between the three groups with a maximum bond strength being found in the control group (Group 3) and the minimum in the bleached group (Group 1). It can be observed that Group 2, which was subjected to antioxidant treatment after bleaching shows an improvement in shear bond strength compared with Group 1.
Statistical Analysis of Data
The data described in [Table 1] was statistically analyzed using one-way analysis of variance (ANOVA) [Table 2] and Tukey honestly significant difference for multiple comparisons [Table 3] and [Table 4].
As per [Table 2], the ANOVA test revealed a significant difference between bond strengths of various samples under study with a significant difference between shear bond strengths of the bleached group (Group 1) ; bleached + antioxidant group (Group 2) and control group (Group 3).
[Table 3] shows that there is a significant difference between the bleached group (Group 1) and control group (Group 3), as well as a significant difference between antioxidant (Group 2) group and control group (Group 3).
The results show that the maximum shear bond strength was found in the control group (Group 3) and the minimum shear bond strength was found in the bleached group (Group 1). The antioxidant group (Group 2) showed higher shear bond strength than the bleached group (Group 1) [Figure 5].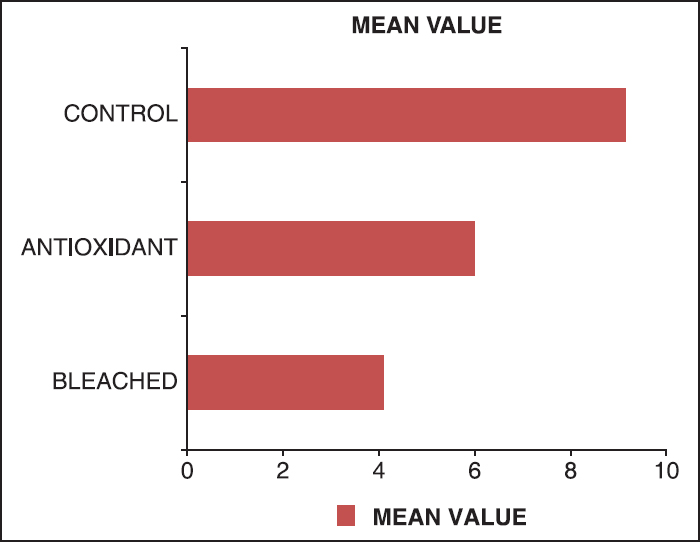 | Figure 5: Representing the mean shear bond strength of various experimental groups
Click here to view |
| Discussion | |  |
For brightening discolored teeth, the use of peroxide releasing agents such as hydrogen peroxide, carbamide peroxide or sodium perborate has become a popular treatment modality, which is comparatively safe. [13]
For effective stain removal, hydrogen peroxide must be able to move through the tooth structure. [13] This is possible because hydrogen peroxide is of low molecular weight and can denature proteins, which increases tissue permeability and allows ions to move through the tooth. However, changes in the enamel structural and morphological integrity induced by bleaching agents is still controversial and causes orthodontists to question whether bleaching adversely affects bond strengths of brackets bonded to enamel. [3],[9],[10] Previous studies claim that the alteration in bond strength due to bleaching might be significant with respect to certain materials such as composite resins and bonding of orthodontic brackets. [13] Studies show that bleaching causes changes in the organic enamel matrix, a loss of calcium and a decrease in micro-hardness that are potential causes for the reduction of bond strength. [6],[13] It is further claimed that residual oxygen, released by the bleaching agent, interferes with resin infiltration and subsequently affects bond strength. [13] The present study concurs with these claims as samples in Group 1 subjected to bleaching showed lowered bond strength compared with the control group. The delay period after bleaching required to obtain bond strengths comparable to that of prebleached enamel is still debated, but the most accepted postbleaching delay is 7 days. [5]
The purpose of this study was to alter the temporary bleaching reduction in bond strength through the application of antioxidant (sodium ascorbate). Ascorbic acid and its salts are potent antioxidants that can be utilized for the purpose of neutralizing the residual oxygen on bleached teeth. [14] The food industry makes wide use of ascorbic acid as an antioxidant since it is nontoxic. Studies claim that sodium ascorbate treatment of bleached teeth before bonding of brackets reduces the reduction in bond strength caused due to bleaching. [11] Application of antioxidant for 10 min was found to be effective and proved beneficial. [15] It was postulated by Lai et al. that an antioxidant in the form of 10% sodium ascorbate permits free radical polymerization of the adhesive resin to proceed without premature termination, which is useful in neutralizing or nullifying the reduction in bond strength due to bleaching. [11]
The results of the present study demonstrate that the antioxidant in the form of sodium ascorbate has a positive effect on the bond strength of previously bleached teeth. [11],[15],[16],[17],[18]
Clinical Relevance and Implications
In view of the increased incidence of adults seeking orthodontic treatment with a previous history of bleaching, this study brings to light the possibility of reversing the reduction in bond strength of orthodontic brackets after bleaching. This would enable orthodontists to avoid the delay in the bonding procedure after bleaching and also enable practitioners to add the aesthetics of bleaching without hampering the mechanical and physical integrity of bond formation.
| References | |  |
| 1. | Sulieman M. An overview of tooth discoloration: Extrinsic, intrinsic and internalized stains. Dent Update 2005;32:463-4, 466-8, 471.  |
| 2. | Türkkahraman H, Adanir N, Güngör AY. Bleaching and desensitizer application effects on shear bond strengths of orthodontic brackets. Angle Orthod 2007;77:489-93.  |
| 3. | Bishara SE, Sulieman AH, Olson M. Effect of enamel bleaching on the bonding strength of orthodontic brackets. Am J Orthod Dentofacial Orthop 1993;104:444-7.  |
| 4. | Cavalli V, Arrais CA, Giannini M, Ambrosano GM. High-concentrated carbamide peroxide bleaching agents effects on enamel surface. J Oral Rehabil 2004;31:155-9.  |
| 5. | Miles PG, Pontier JP, Bahiraei D, Close J. The effect of carbamide peroxide bleach on the tensile bond strength of ceramic brackets: An in vitro study. Am J Orthod Dentofacial Orthop 1994;106:371-5.  |
| 6. | Titley K, Torneck CD, Smith DC. Effect of concentrated hydrogen peroxide solution on the surface morphology of cut human dentin. J Endod 1988;14:69-74.  |
| 7. | Ruse ND, Smith DC, Torneck CD, Titley KC. Preliminary surface analysis of etched, bleached, and normal bovine enamel. J Dent Res 1990;69:1610-3.  |
| 8. | Josey AL, Meyers IA, Romaniuk K, Symons AL. The effect of a vital bleaching technique on enamel surface morphology and the bonding of composite resin to enamel. J Oral Rehabil 1996;23:244-50.  |
| 9. | Uysal T, Basciftci FA, Usümez S, Sari Z, Buyukerkmen A. Can previously bleached teeth be bonded safely? Am J Orthod Dentofacial Orthop 2003;123:628-32.  |
| 10. | Bishara SE, Oonsombat C, Soliman MM, Ajlouni R, Laffoon JF. The effect of tooth bleaching on the shear bond strength of orthodontic brackets. Am J Orthod Dentofacial Orthop 2005;128:755-60.  |
| 11. | Lai SC, Tay FR, Cheung GS, Mak YF, Carvalho RM, Wei SH, et al. Reversal of compromised bonding in bleached enamel. J Dent Res 2002;81:477-81.  |
| 12. | Cavalli V, Reis AF, Giannini M, Ambrosano GM. The effect of elapsed time following bleaching on enamel bond strength of resin composite. Oper Dent 2001;26:597-602.  |
| 13. | Dishman MV, Covey DA, Baughan LW. The effects of peroxide bleaching on composite to enamel bond strength. Dent Mater 1994;10:33-6.  |
| 14. | Carr AC, Tijerina T, Frei B. Vitamin C protects against and reverses specific hypochlorous acid- and chloramine-dependent modifications of low-density lipoprotein. Biochem J 2000;346:491-9.  |
| 15. | Bulut H, Kaya AD, Turkun M. Tensile bond strength of brackets after antioxidant treatment on bleached teeth. Eur J Orthod 2005;27:466-71.  |
| 16. | Garcia EJ, Mena-Serrano A, de Andrade AM, Reis A, Grande RH, Loguercio AD. Immediate bonding to bleached enamel treated with 10% sodium ascorbate gel: A case report with one-year follow-up. Eur J Esthet Dent 2012;7:154-62.  |
| 17. | Kunt GE, Yýlmaz N, Sen S, Dede DÖ. Effect of antioxidant treatment on the shear bond strength of composite resin to bleached enamel. Acta Odontol Scand 2011;69:287-91.  |
| 18. | Kaya AD, Türkün M, Arici M. Reversal of compromised bonding in bleached enamel using antioxidant gel. Oper Dent 2008;33:441-7.  |
[Figure 1], [Figure 2], [Figure 3], [Figure 4], [Figure 5]
[Table 1], [Table 2], [Table 3], [Table 4]
|