|


 |
| REVIEW ARTICLE |
|
| Year : 2011 | Volume
: 3
| Issue : 1 | Page : 13-16 |
|
|
Split calvarial bone graft for the reconstruction of skull defects
Amit Agrawal1, Lakshmi N Garg2
1 Department of Neurosurgery, MM Institute of Medical Sciences and Research, Mullana (Ambala), Haryana, India
2 Department of ENT, MM Institute of Medical Sciences and Research, Mullana (Ambala), Haryana, India
| Date of Web Publication | 30-Mar-2011 |
Correspondence Address:
Amit Agrawal
Department of Neurosurgery, MM Institute of Medical Sciences and Research, Mullana, Ambala - 133203, Haryana
India
 Source of Support: None, Conflict of Interest: None  | 12 |
DOI: 10.4103/2006-8808.78465

 Abstract Abstract | | |
Cranioplasty is a common, but formidable surgical procedure for neurosurgeons, in patients with scalp and / or calvarial defects. This procedure can be simple or complex. The main objectives of cranioplasty are: To achieve primary wound healing, obliterate dead space, and seal off sterile cranial areas from contaminated oronasal cavities, to restore the normal barriers protecting the intracranial structures (together with a satisfactory cosmetic result) and obtain a permanent or very durable reconstruction, using biologically inert materials, and also to restore the aesthetics. The greatest problem is selecting the optimum material for repair of the cranial defect. Many synthetic substitutions of the dura and bone are often used for reconstruction of the skull base; unfortunately, these methods bear significant disadvantages and can induce chronic inflammation, carry a high risk of infection, and are inferior to biological sources in terms of strength and sealing quality [with the exception of some materials, such as titanium mashes and CortossTM (Orthovita® , Malvern, USA), which are seen to have more strength than the thin split thickness calvarial bone]. The primary aim of this article is to review the basic principles to use the split calvarial graft for the reconstruction of the skull defect. Keywords: Autologous bone, bone graft, calvarial bone splitting, calvarial defect, cranioplasty
How to cite this article:
Agrawal A, Garg LN. Split calvarial bone graft for the reconstruction of skull defects. J Surg Tech Case Report 2011;3:13-6 |
How to cite this URL:
Agrawal A, Garg LN. Split calvarial bone graft for the reconstruction of skull defects. J Surg Tech Case Report [serial online] 2011 [cited 2016 Jun 12];3:13-6. Available from: http://www.jstcr.org/text.asp?2011/3/1/13/78465 |
 Introduction Introduction | |  |
Cranioplasty is a common, but formidable surgical procedure for neurosurgeons, in patients with scalp and/or calvarial defects. This procedure can be simple or complex. [1],[2] There is evidence of cranioplasty having been performed by several early cultures, including pre-Columbian Incans, using gold or silver plates, and by neolithic Celts using bone 'rondelles'. [3] However, the first reported cranioplasty was probably that of a Russian nobleman who, after receiving a sword blow to the head, had the resultant defect (and his health) restored with a piece of dog's cranium (Van Meekeren, 1668). Subsequently, after he had been excommunicated from the Russian church (which could not accept the presence of animal bone on a human skull), removal of the graft was impossible, due to bone union. [3] The primary aim of this article is to review the basic principles, to use the split calvarial graft for the reconstruction of a skull defect.
 Aim of Surgery Aim of Surgery | |  |
The main objectives of cranioplasty are: To achieve primary wound healing, obliterate dead space, and seal off sterile cranial areas from contaminated oronasal cavities, to restore the normal barriers protecting the intracranial structures and obtain a permanent or very durable reconstruction using biologically inert materials, [1],[4],[5] and also to restore the esthetics. [5],[6],[7],[8]
 Indications Indications | |  |
Pathological defects or alterations in the shape of the calvarium may be caused by a number of processes, including traumatic defects, resection of benign or malignant tumors, congenital lesions, and iatrogenic injuries, out of these more common causes of skull defects, including trauma, neurosurgical procedures, and infections. [1],[2],[5],[9],[10],[11],[12],[13],[14],[15] Most calvarial reconstructions are performed immediately unless the wound is infected. If infection is present, the reconstruction must be delayed until the infection has been treated. [16]
 Material Choice Material Choice | |  |
Reconstruction of skull defects is technically challenging, but can be achieved with the use of biological tissue, such as the split calvarial bone graft or posterior wall of the sinus or iliac crest, or with artificial materials, such as the 3D titanium mesh. [5],[17],[18] The ideal substitute for undertaking cranioplasty must be biocompatible, strong, and lightweight; it must be malleable, to precisely fit even complicated cranial defects, nonmagnetic, chemically inert; radiolucent; non-ferromagnetic; readily available; inexpensive and easily secured, and must have long-term stability. However, no such material currently exists, making natural bone the obvious choice to be used as cranioplasty material. [18] Alloplastic implants have the advantage of being readily available, easy to handle and shape, and undergo minimal resorption, however, alloplastic implants are permanent foreign bodies that are susceptible to infection and exposure over time. The advantages of reconstruction with autologous bone include a lower incidence of graft loss than occurs with alloplastic material. Also, exposure and infection of the autologous bone can sometimes be managed without complete graft loss, whereas, when alloplastic materials become exposed or infected, often the only choice is removal of the foreign material, [19] (exceptions are porous polyethylene sheet (Medpor) allografts, as their infection can be managed by intravenous antibiotics). [20],[21],[22],[23] Furthermore, the mechanical, immunological, and technical-grafting properties of autologous bone, together with its superior esthetic, and psychological effects, probably make it the best material for cranioplasty. [1]
 Technique Technique | |  |
The split calvarial bone technique was popularized by Tessier in 1932, [24] and first applied in nasal bone reconstruction by Jackson et al. [25] The preferred skin incision is generally the coronal one, as it provides a wide exposure of the skull surface and may often proffer simultaneous visualization of the bony defect and the area identified for harvest of the graft. [1],[12],[13],[26] After a bicoronal incision, the scalp flap is raised, a plane is created between the pericranium and skull periosteum, and the region that requires cranioplasty is prepared. Any dural dehiscence or loss is first repaired, grafted if required, and can be sealed with fibrin glue or an equivalent. The external surface of the skull is then exposed by subgaleal dissection. Subsequently, the outlines of the cranial defect are traced onto a sheet of transparent plastic and then transferred onto the surface of the skull, chosen as a harvest area. The next step is resection of the graft for which several different techniques can be employed. [1],[2],[26],[27],[28] A bone flap of the same size is removed from the skull and the outer and inner tables can be split apart and used to reconstruct the defect or defects. For this technique a section of the donor skull to be used is split and the outer table is applied to cover the craniotomy defect, leaving the inner table to cover the donor site. Split calvarial grafts result in an aesthetically pleasing contour. [12],[13],[29] Larger, split-thickness cranial bone grafts can be used to replace numerous smaller fracture fragments, greatly facilitating the fixation device application and providing much thicker scaffolding, which will better maintain soft tissue contour during remodeling and new bone formation. [13],[30] If possible a single-stage procedure will allow the patient to undergo only one procedure rather than two or more staged operations and avoid several weeks with a substantial bony defect. [11] Calvarial bone grafts have the benefit of being harvested from the same operative field as the defect. Split outer table grafts are taken from the parietal region of the skull, posterior to the coronal suture, where the skull is the thickest [Figure 1] and [Figure 2]. The graft must not be harvested in the midline because of the risk of injuring the sagittal sinus. [19]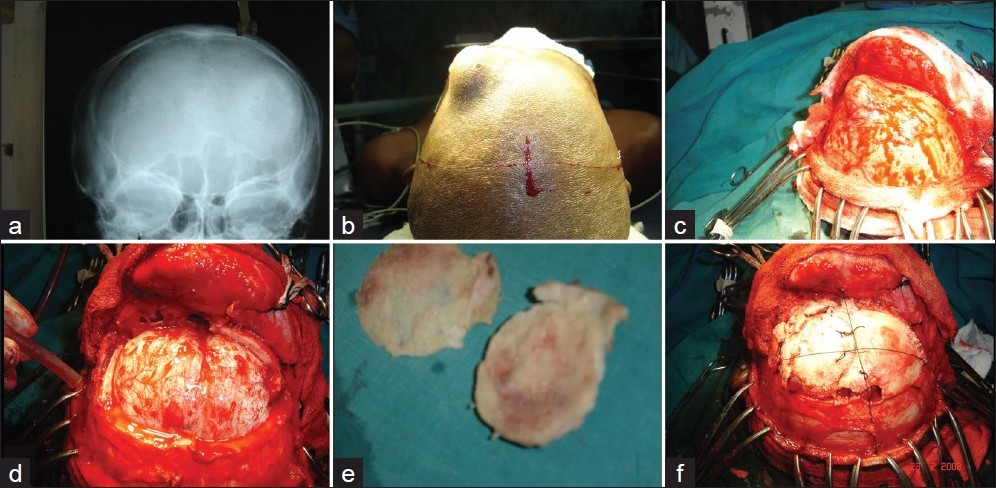 | Figure 1: Treatment of patient with a massive osteoma of the left frontal bone
Click here to view |
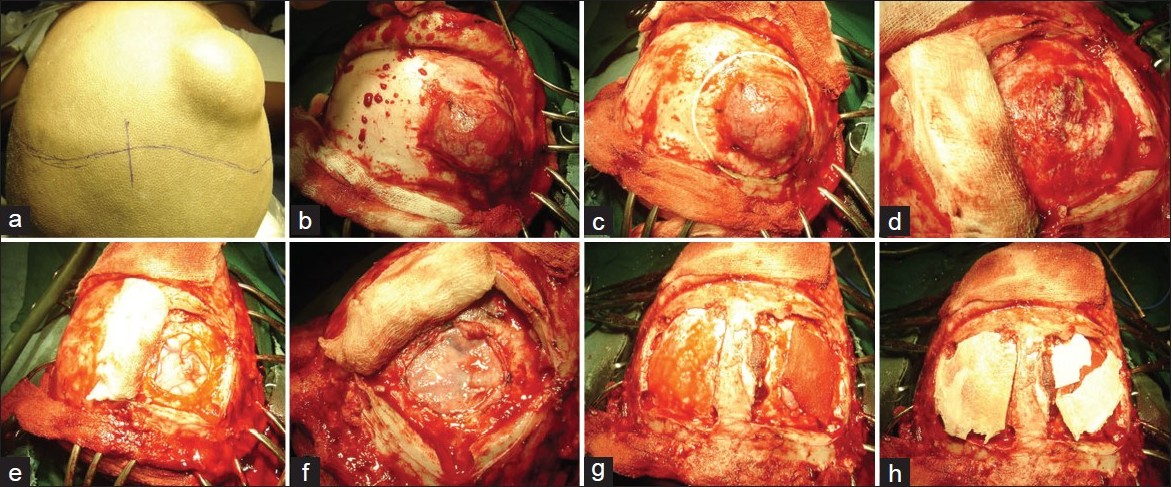 | Figure 2: A case of large Ewing's sarcoma involving the right frontal bone
Click here to view |
 Advantages Advantages | |  |
Although calvarial bone grafts are used today for various scalp defect reconstructions, the complication rates related to the bone graft are surprisingly low. [10] Many studies have reported no resorption and no loss of calvarial transplants after repair, on a short-term follow-up, ranging from 1 to 3.7 years. [31],[32],[33],[34],[35] There is almost a unanimous agreement that autogenous calvarium possesses far better characteristics and quality than the most widely used alloplastic materials currently available. [1] There is no type of metal, acrylic resin, or any other type of alloplastic material that fulfills this wide range of requisites, and it is much simpler, less expensive, and safer for the patient to use autologous bone whenever possible. [1],[12],[13],[15],[36] Apart from this, fresh autologous bone is the most suitable material for reconstruction of cranial defects in view of its perfect histocompatibility, optimal mechanical properties, and good anatomofunctional fusion of the graf with the adjacent bone, as well as the possibility of partial or total revitalization of the graft itself. [1] Live tissue is biologically active and fuses quickly with the adjacent bone, giving excellent results. [1] Also, the autologous bone ensures the best possible physiological and cosmetic results, autologous bone grafts usually display bone regeneration processes, do not have a foreign body reaction, and present a low incidence of infection. [37] In addition, calvarial bone grafts in the pediatric age group are fairly malleable, allowing reconstructive surgeons to reproduce the precise contour of the calvarium with relative ease. [18]
 Disadvantage Disadvantage | |  |
The greatest problem is selecting the optimum material for repair of the cranial defect. [2] Many synthetic substitutions of dura and bone are often used for reconstruction of the skull base; unfortunately, these methods bear significant disadvantages and can induce chronic inflammation, carry a high risk of infection, and are inferior to the biological sources in terms of strength and sealing quality, [38],[39] [with the probable exception of some materials, such as titanium mashes and CortossTM (Orthovita®, Malvern, USA), which prove to have more strength than the thin split thickness calvarial bone]. [40],[41],[42]
A disadvantage of calvarial grafts is the limited size of the graft available, particularly when the defect is adjacent to the graft donor site. Another disadvantage of calvarial grafts is the risk of violating the inner table or dura during harvest. [19] Correction of large calvarial defects with autografts may also be quite time consuming, [3],[43] Moreover, both the donor and recipient sites are less biomechanically stable than the adjacent skull. [3] Splitting of the bone requires experience, as sometimes the bone cracks into several pieces. However, the pieces of bone can easily be fixed with miniplates and screws. Therefore, splitting of the complete bone is not necessary. [2] Other complications specific to the bone relate to its harvest: split calvarial grafts carry the risk of intracerebral hematoma, subarachnoid hemorrhage, dural tears, and CSF leaks. [1],[2],[16]
 Conclusion Conclusion | |  |
In summary, calvarial bone grafts are used today for various scalp defect reconstructions, with incidence of complication rates related to the bone graft being surprisingly low. As no specific cost analysis has been attempted, it is difficult to compare whether the added cost of the synthetic material would be offset by the time saved in the operating room compared to calvarial bone grafting and the subsequent rigid fixation.
 References References | |  |
| 1. | Artico M, Ferrante L, Pastore FS, Ramundo EO, Cantarelli D, Scopelliti D, et al. Bone autografting of the calvaria and craniofacial skeleton: Historical background, surgical results in a series of 15 patients, and review of the literature. Surg Neurol 2003;60:71-9. 
[PUBMED] [FULLTEXT] |
| 2. | Inoue A, Satoh S, Sekiguchi K, Ibuchi Y, Katoh S, Ota K, et al. Cranioplasty with split-thickness calvarial bone. Neurol Med Chir (Tokyo) 1995;35:804-7. 
[PUBMED] [FULLTEXT] |
| 3. | Sanan A, Haines SJ. Repairing holes in the head: A history of cranioplasty. Neurosurgery 1997;40:588-603. 
|
| 4. | Neligan PC, Boyd JB. Reconstruction of the cranial base defect. Clin Plast Surg 1995;22:71-7. 
[PUBMED] |
| 5. | Gil Z, Abergel A, Leider-Trejo L, Khafif A, Margalit N, Amir A, et al. A comprehensive algorithm for anterior skull base reconstruction after oncological resections. Skull Base 2007;17:25-37. 
[PUBMED] [FULLTEXT] |
| 6. | Erculei F, Walker AE. Posttraumatic Epilepsy and Early Cranioplasty. J Neurosurg 1963;20:1085-9. 
[PUBMED] [FULLTEXT] |
| 7. | Tabaddor K, LaMorgese J. Complication of a large cranial defect. Case report. J Neurosurg 1976;44:506-8. 
[PUBMED] [FULLTEXT] |
| 8. | Lee KB, Ang ES, Tan KC. Reconstructive challenges in the management of a rare case of sphenoid osteosarcoma--a case report. Singapore Med J 2001;42:586-9. 
[PUBMED] |
| 9. | Guy L, William L. Complications using grafts and implants in rhinoplasty. Head Neck Surg 2007;18:315-23. 
|
| 10. | Smolka W, Eggensperger N, Kollar A, Iizuka T. Midfacial reconstruction using calvarial split bone grafts. Arch Otolaryngol Head Neck Surg 2005;131:131-6. 
[PUBMED] [FULLTEXT] |
| 11. | Ismail N, Shehu B, Lasseini A, Hassan I, Shilong D, Obande J, et al. Solitary giant neurofibroma of the scalp with calvarial defect in a child. J Surg Tech Case Report 2010;2:24-6. 
 |
| 12. | Agrawal A, Dulani R, Mahadevan A, Vagaha SJ, Vagha J, Shankar SK. Primary Ewing's sarcoma of the frontal bone with intracranial extension. J Cancer Res Ther 2009;5:208-9. 
|
| 13. | Agrawal A, Rao KS, Krishnamoorthy B, Shetty RB, Anand M, Jain H. Single stage craniofacial reconstruction for fronto-nasal encephalocele and hypertelorism in an adult. Singapore Med J 2007;48:e215-9. 
[PUBMED] [FULLTEXT] |
| 14. | Desai KI, Nadkarni TD, Bhayani RD, Goel A. Intradiploic meningioma of the orbit: A case report. Neurol India 2004;52:380-2. 
[PUBMED]  |
| 15. | Rajendra PB, Mathew TP, Agrawal A, Sabharawal G. Characteristics of associated craniofacial trauma in patients with head injuries: An experience with 100 cases. J Emerg Trauma Shock 2009;2:89-94. 
[PUBMED]  |
| 16. | Lin SJ, Hanasono MM, Skoracki RJ. Scalp and calvarial reconstruction. Semin Plast Surg 2008;22:281-93. 
[PUBMED] [FULLTEXT] |
| 17. | Ilankovan V, Jackson IT. Experience in the use of calvarial bone grafts in orbital reconstruction. Br J Oral Maxillofac Surg 1992;30:92-6. 
[PUBMED] |
| 18. | Ducic Y. Titanium mesh and hydroxyapatite cement cranioplasty: A report of 20 cases. J Oral Maxillofac Surg 2002;60:272-6. 
[PUBMED] [FULLTEXT] |
| 19. | Earley MJ, Green MF, Milling MA. A critical appraisal of the use of free flaps in primary reconstruction of combined scalp and calvarial cancer defects. Br J Plast Surg 1990;43:283-9. 
[PUBMED] |
| 20. | Emsen IM. E-M shaped septal encircling with Medpor reconstruction on crooked noses: Personal technique and postoperative results. J Craniofac Surg 2008;19:216-26. 
[PUBMED] [FULLTEXT] |
| 21. | Deshpande S, Munoli A. Long-term results of high-density porous polyethylene implants in facial skeletal augmentation: An Indian perspective. Indian J Plast Surg 2010;43:34-9. 
[PUBMED]  |
| 22. | Couldwell WT, Stillerman CB, Dougherty W. Reconstruction of the skull base and cranium adjacent to sinuses with porous polyethylene implant: Preliminary report. Skull Base Surg 1997;7:57-63. 
[PUBMED] [FULLTEXT] |
| 23. | Cenzi R, Farina A, Zuccarino L, Carinci F. Clinical outcome of 285 Medpor grafts used for craniofacial reconstruction. J Craniofac Surg 2005;16:526-30. 
[PUBMED] [FULLTEXT] |
| 24. | Tessier P. Autogenous bone grafts taken from the calvarium for facial and cranial applications. Clin Plast Surg 1982;9:531-8. 
[PUBMED] |
| 25. | Jackson IT, Smith J, Mixter RC. Nasal bone grafting using split skull grafts. Ann Plast Surg 1983;11:533-40. 
[PUBMED] |
| 26. | Santonirugiu P. Repair of skull defects by outer table osteoperiosteal free grafts. Plast Reconstr Surg 1969;43:157-61. 
|
| 27. | Psillakis JM, Nocchi VL, Zanini SA. Repair of large defect of frontal bone with free graft of outer table of parietal bones. Plast Reconstr Surg 1979;64:827-30. 
[PUBMED] |
| 28. | Casanova R, Cavalcante D, Grotting JC, Vasconez LO, Psillakis JM. Anatomic basis for vascularized outer-table calvarial bone flaps. Plast Reconstr Surg 1986;78:300-8. 
[PUBMED] |
| 29. | Borkar SA, Tripathi AK, Satyarthee GD, Rishi A, Kale SS, Sharma BS. Fronto-orbital intradiploic transitional meningioma. Neurol India 2008;56:205-6. 
[PUBMED]  |
| 30. | Chou TD, Lee WT, Chen SL, Lee CH, Chen SG, Chen TM, et al. Split calvarial bone graft for chemical burn-associated nasal augmentation. Burns 2004;30:380-5. 
[PUBMED] [FULLTEXT] |
| 31. | Gruss JS, Mackinnon SE, Kassel EE, Cooper PW. The role of primary bone grafting in complex craniomaxillofacial trauma. Plast Reconstr Surg 1985;75:17-24. 
[PUBMED] |
| 32. | Hunter D, Baker S, Sobol SM. Split calvarial grafts in maxillofacial reconstruction. Otolaryngol Head Neck Surg 1990;102:345-50. 
[PUBMED] |
| 33. | Frodel JL. Calvarial bone graft harvest in children. Otolaryngol Head Neck Surg 1999;121:78-81. 
[PUBMED] |
| 34. | Brusati R, Biglioli F, Mortini P, Raffaini M, Goisis M. Reconstruction of the orbital walls in surgery of the skull base for benign neoplasms. Int J Oral Maxillofac Surg 2000;29:325-30. 
[PUBMED] [FULLTEXT] |
| 35. | Powell NB, Riley RW. Facial contouring with outer-table calvarial bone. A 4-year experience. Arch Otolaryngol Head Neck Surg 1989;115:1454-8. 
[PUBMED] [FULLTEXT] |
| 36. | Hayward RD. Cranioplasty: Don't forget the patient's own bone is cheaper than titanium. Br J Neurosurg 1999;13:490-1. 
[PUBMED] |
| 37. | Acikgoz B, Ozcan OE, Erbengi A, Bertan V, Ruacan S, Acikgoz HG. Histopathologic and microdensitometric analysis of craniotomy bone flaps preserved between abdominal fat and muscle. Surg Neurol 1986;26:557-61. 
|
| 38. | Simpson D, Robson A. Recurrent subarachnoid bleeding in association with dural substitute. Report of three cases. J Neurosurg 1984;60:408-9. 
[PUBMED] [FULLTEXT] |
| 39. | Neligan PC, Mulholland S, Irish J, Gullane PJ, Boyd JB, Gentili F, et al. Flap selection in cranial base reconstruction. Plast Reconstr Surg 1996;98:1159-66. 
[PUBMED] [FULLTEXT] |
| 40. | Pomrink GJ, DiCicco MP, Clineff TD, Erbe EM. Evaluation of the reaction kinetics of CORTOSS, a thermoset cortical bone void filler. Biomaterials 2003;24:1023-31. 
[PUBMED] [FULLTEXT] |
| 41. | Ozlen F, Abuzayed B, Dashti R, Isler C, Tanriover N, Sanus GZ. Low-profile 1-piece bifrontal craniotomy for anterior skull base approach and reconstruction. J Craniofac Surg 2010;21:233-8. 
[PUBMED] [FULLTEXT] |
| 42. | Sanus GZ, Tanriverdi T, Ulu MO, Kafadar AM, Tanriover N, Ozlen F. Use of Cortoss as an alternative material in calvarial defects: The first clinical results in cranioplasty. J Craniofac Surg 2008;19:88-95. 
[PUBMED] [FULLTEXT] |
| 43. | Bite U, Jackson IT, Wahner HW, Marsh RW. Vascularized skull bone grafts in craniofacial surgery. Ann Plast Surg 1987;19:3-15. 
[PUBMED] |
[Figure 1], [Figure 2]
| This article has been cited by | | 1 |
Scapular Bone Grafts |
|
| Aydin Turan,Naci Kostakoglu,Umut Tuncel,Erkan Gökçe,Fatma Markoç | | Annals of Plastic Surgery. 2016; 76(5): 509 | | [Pubmed] | [DOI] | | | 2 |
Scalp reconstruction |
|
| Raj Dedhia,Quang Luu | | Current Opinion in Otolaryngology & Head and Neck Surgery. 2015; 23(5): 407 | | [Pubmed] | [DOI] | | | 3 |
Bone morphogenetic protein-9 effectively induces osteogenic differentiation of reversibly immortalized calvarial mesenchymal progenitor cells |
|
| Chad M. Teven,Michael T. Rossi,Deana S. Shenaq,Guillermo A. Ameer,Russell R. Reid | | Genes & Diseases. 2015; 2(3): 268 | | [Pubmed] | [DOI] | | | 4 |
Cranioplasty Using a Modified Split Calvarial Graft Technique in Cleidocranial Dysplasia |
|
| Young Taek Jung,Jae Ik Cho,Sang Pyung Lee | | Journal of Korean Neurosurgical Society. 2015; 58(1): 79 | | [Pubmed] | [DOI] | | | 5 |
Reconstructive approach to hostile cranioplasty: A review of the University of Chicago experience |
|
| Abigail J. Fong,Benjamin T. Lemelman,Sandi Lam,Grant M. Kleiber,Russell R. Reid,Lawrence J. Gottlieb | | Journal of Plastic, Reconstructive & Aesthetic Surgery. 2015; 68(8): 1036 | | [Pubmed] | [DOI] | | | 6 |
A comparison between autograft alone, bone cement, and demineralized bone matrix in cranioplasty |
|
| Ann W. Plum,Sherard A. Tatum | | The Laryngoscope. 2015; 125(6): 1322 | | [Pubmed] | [DOI] | | | 7 |
Changes in Graft Thickness After Skull Defect Reconstruction With Autogenous Split Calvarial Bone Graft |
|
| Tack Jin Chang,Jong Woo Choi,Young Shin Ra,Seok Ho Hong,Young Hyun Cho,Kyung Suk Koh | | Journal of Craniofacial Surgery. 2014; 25(4): 1241 | | [Pubmed] | [DOI] | | | 8 |
Clinical Outcomes in Cranioplasty |
|
| Sashank Reddy,Saami Khalifian,José M. Flores,Justin Bellamy,Paul N. Manson,Eduardo D. Rodriguez,Amir H. Dorafshar | | Plastic and Reconstructive Surgery. 2014; 133(4): 864 | | [Pubmed] | [DOI] | | | 9 |
Lid Cranioplasty |
|
| Jyoshid R. Balan,Satyaswarup Tripathy | | The Journal of Craniofacial Surgery. 2014; : 1 | | [Pubmed] | [DOI] | | | 10 |
ing the head as a mould for cranioplasty with methylmethacrylate |
|
| Bot, G.M., Ismail, N.J., Usman, B., Sale, D., Shehu, B.B. | | Journal of Neurosciences in Rural Practice. 2013; 4(4): 471-474 | | [Pubmed] | | | 11 |
Use of autologous comminuted calvarial fragments and pedicled pericranial graft for single stage repair of frontal and cranial base injury |
|
| Amit Agrawal,Surya Pratap Singh | | The Indian Journal of Neurotrauma. 2013; 10(1): 48 | | [Pubmed] | [DOI] | | | 12 |
Reconstruction of cranial defects with individually formed cranial prostheses made of polypropylene polyester knitwear: An analysis of 48 consecutive patients |
|
| Kasprzak, P. and Tomaszewski, G. and Kotwica, Z. and Kwinta, B. and ZwoliÅski, J. | | Journal of Neurotrauma. 2012; 29(6): 1084-1089 | | [Pubmed] | | | 13 |
Reconstruction of Cranial Defects with Individually Formed Cranial Prostheses Made of Polypropylene Polyester Knitwear: An Analysis of 48 Consecutive Patients |
|
| Piotr Kasprzak,Grzegorz Tomaszewski,Zbigniew Kotwica,Borys Kwinta,Jerzy Zwolinski | | Journal of Neurotrauma. 2012; 29(6): 1084 | | [Pubmed] | [DOI] | |
|




 |
 |
|