|
 
 |
| ORIGINAL ARTICLE |
|
| Year : 2015 | Volume
: 10
| Issue : 1 | Page : 1-6 |
|
Overall survival of females with breast cancer in the National Cancer Institute, University of Gezira, Sudan
Ahmed M Elhaj1, AI Abdalsalam2, AO Abuidris1, AA Eltayeb1
1 Department of Oncology, National Cancer Institute, University of Gezira, Wad Madani, Sudan
2 Department of Non-Communicable Diseases, Federal Ministry of Health, Khartoum, Sudan
| Date of Web Publication | 21-May-2015 |
Correspondence Address:
Ahmed M Elhaj
Department of Oncology, National Cancer Institute, University of Gezira, Wad Madani
Sudan
  | Check |
DOI: 10.4103/1858-5000.157499 
Background: Breast cancer represents 20% of all cancer cases registered in the National Cancer Institute (NCI), University of Gezira, Sudan. New cases of breast cancer presented during the period from January 2001 to December 2001 were included in this study. The objective was to estimate the prognosis of breast cancer in (NCI) in terms of 5 years survival. Methods: The data were collected mainly from the hospital records. In cases of missed data, addresses of kin provided in the hospital records were contacted to find out the survival status of the patients. Data were analyzed according to two sets of variables; patients' related variables and tumor-related variables. Kaplan-Meier method was used to determine the overall survival curve. Results: Totally, 64 patients were evaluated for the study. Of them, 41 (64%) patients presented with disease stage 3 and 4. The mean of follow-up duration was 21.5 months, while the median was 10.5 months. The total number of deaths was 41; those who died in the hospital were 6, representing only 14.6% of the total deaths. The median overall survival period for the population of the study was 40 months and the cumulative survival probability was 38%. Conclusion: The majority of the patients who presented with advanced-stage disease seem to account for the poor overall survival reported in this study. Early detection of breast cancer by breast self-examination and physician breast examination should be encouraged in Sudan to improve treatment results in breast cancer. Keywords: Breast cancer, overall survival, Sudan
How to cite this article:
Elhaj AM, Abdalsalam A I, Abuidris A O, Eltayeb A A. Overall survival of females with breast cancer in the National Cancer Institute, University of Gezira, Sudan. Sudan Med Monit 2015;10:1-6 |
How to cite this URL:
Elhaj AM, Abdalsalam A I, Abuidris A O, Eltayeb A A. Overall survival of females with breast cancer in the National Cancer Institute, University of Gezira, Sudan. Sudan Med Monit [serial online] 2015 [cited 2018 Sep 5];10:1-6. Available from: http://www.sudanmedicalmonitor.org/text.asp?2015/10/1/1/157499 |
| Introduction | |  |
Breast cancer constitutes a major public health issue globally with over 1 million new cases diagnosed annually, resulting in over 400,000 annual deaths and about 4.4 million women living with the disease. It is the most common site of specific malignancy affecting women and the most common cause of cancer mortality in women worldwide. [1],[2],[3] There is an international/geographical variation in the incidence of breast cancer. Incidence rates are higher in the developed countries than in the developing countries and Japan. Incidence rates are also higher in urban areas than in the rural areas. [2] In Africa, breast cancer has overtaken cervical cancer as the most common malignancy affecting women and the incidence rates appear to be rising. [4],[5] These increases in incidence are due to changes in the demography, socioeconomic parameters, epidemiologic risk factors, better reporting, and awareness of the disease. While mortality rates are declining in the developed world (Americas, Australia, and Western Europe) as a result of screening, early diagnosis, and improved cancer treatment programs, the converse is true in the developing world as well as in eastern and Central Europe , [2],[6] this may be due to lack of screening programs, and advanced disease at presentation. The hallmarks of the disease in Africa are patients presenting at advanced-stage, lack of adequate mammography screening programs, preponderance of younger premenopausal patients, and consequently a high morbidity and mortality. [2],[5],[7],[8],[9],[10],[11],[12],[13],[14] Like all other diseases breast cancer in African population does not receive due attention by the health care providers. Breast cancer in African people has been reported to present in the late stage. However, this perception has never been studied using a standard classification like the tumor, node, metastasis (TNM) classification of International Union against Cancer. [15]
In Sudan, there is no National Cancer Registry. According to the Federal Ministry of Health (FMOH) reports in 2000, cancer was the third leading cause of death after malaria and viral pneumonia, accounting for 5% of all deaths. In women, breast, cervical, and ovarian cancer remained the three most common cancers. [16] A major challenge to the treatment of cancer in Sudan, as in most developing countries, is that most patients present with advanced-stage disease. A total of 78% of Sudanese breast cancer patients have stage III or IV disease (TNM classification) when they first seek medical treatment. [16],[17] In these stages, treatment may often involve multiple modalities, including surgery, radiotherapy, chemotherapy, and hormone therapy, and has a markedly diminished chance of success. Therefore, there is an urgent need for better early detection of cancer in Sudan to make treatment more effective, less costly, less invasive, and more accessible and acceptable to patients. In many countries, cancer may carry a stigma that prevents people from seeking medical care in the early stages of the disease. In Sudan, there is a common perception that cancer is transmissible, leading to isolation of patients and breakdown of marriages, which may lead patients to hesitate to seek proper care. Ignorance about the signs and symptoms of cancer also means that many people do not present until they have very advanced disease. Hence, much is needed to be done in the area of health education and screening of cancer. [16]
In Sudan, there are only two reference cancer centers, both located in Central Sudan, that is, the Radiation and Isotopes Center, Khartoum (RICK) and the National Cancer Institute of the University of Gezira (NCI-UG) in Wad Madani. Breast cancer accounts for about one-fifth of all treated cancers and is the most frequent malignancy seen at both RICK (17%, i.e. 2395 ⁄ 13,924 recorded cancer cases) and NCI-UG (21%, i.e. 732 ⁄ 3547 recorded cancer cases). [2],[3],[17],[18],[19],[20],[21],[22],[23] This might partly reflect awareness bias, as breast masses or ulcerated lesions are readily evident to the patients themselves, as well as hospitalization bias, given the fact that radiotherapy facilities at RICK, and NCI-UG are used to treat advanced breast cancer.
Since no study was conducted to assess the overall survival of breast cancer patients in the region of Central Sudan, a study to assess this aspect is thought necessary to determine the prognosis of patients treated. The study also may furnish grounds for further studies and monitoring of improvement of patients management in the future taking in consideration that the selected study group represent the first group of patient, who had been treated before adopting a unified treatment guidelines in Gezira area of Central Sudan.
| Methods | |  |
The study was a retrospective analytical study based on data collected from patients' hospital files and addresses. The study addressed known cases of female breast cancer who presented to the NCI during the period from January 2001 to December 2001. The size of the study population was 66 patients (32% of all cancer cases registered during the study period). All patients were referred from surgical units in different hospitals. The overall survival was calculated from the date of presentation (which doesn't mean the beginning of the disease) to oncology department to the date of death or last follow-up. Two patients were excluded from the analysis because one of them was found to have two files, and the other file contained irrelevant documents. The data were collected mainly from the hospital records. In the event of missing data, addresses of the kin provided in the hospital record were contacted to find out the survival status of the patient. Variables collected from the patients' files concerning their characteristics and the characteristics of their disease. The information analyzed for each patient included body mass index, age, parity. Further information about breast tumor (e.g., stage, grade, lymph node status) was also collected and analyzed. Data were analyzed using Statistical Package for Social Sciences (SPSS version 13, SPSS Inc) according to two sets of variables; patients' related variables and tumor' related variables. Chi-square (χ²) distribution was utilized to assess the statistical significance of the effect of each variable on overall survival. Kaplan-Meier method of survival analysis was used to determine the overall survival curve in relation to the variables. The Kaplan-Meier method is a nonparametric technique for estimating time-related events (the survivorship function). Usually, it is used to analyze death as an outcome. It may be used effectively to analyze time to an endpoint. The Kaplan-Meier analysis allows estimation of survival over time, even when patients drop out or are studied for different lengths of time. In it, the exact point in time when each death occurred is identified, so that each death terminates the previous interval and a new interval is started. [24] Log rank was used to show comparability in survival between groups of the same variable.
| Results | |  |
The characteristics of the study population are shown in [Table 1]. Six patients (9.1%) of the total study population were not seen after the first visit. Other 2 (3%) patients followed up for 1-month, while those followed for 2 months were 7 (10%) and those followed for 60 months were 11 (16.7%). The rest had different follow-up durations. The mean of follow-up duration in this study was 21.5 months, while the median was 10.5 months. The total number of deaths was 41; those who died in the hospital were 6, representing only 14.6% of the total deaths. A middle age patient (40-50 years) showed better survival in both median survival period (53 months) and cumulative survival probability (40%). The cumulative survival probability decreased with advanced disease, (100% for stage I, and only 5% for stage IV). Poor tumor grading carried poor cumulative survival probability (28%). Negative lymph node status showed better survival in term of both median survival period (47 months) and cumulative survival probability (48%). Thin and overweight patients had equal median survival period of 26 months. Thin patients had 50% cumulative survival probability. Having more children is a favorable factor for survival, the cumulative survival probability was 40% for multiparous. Chi-square analysis showed that all variables studied were statistically not significant in affecting survival and this could be explained by the small study population. Using log rank test, the difference between stages and grade groups were the only statistically significant factors [Table 2].
Assessment of the study population overall survival indicates that 50% of them were expected to die after 40 months. The 5 years survival probability of the study population was 38% [Figure 1].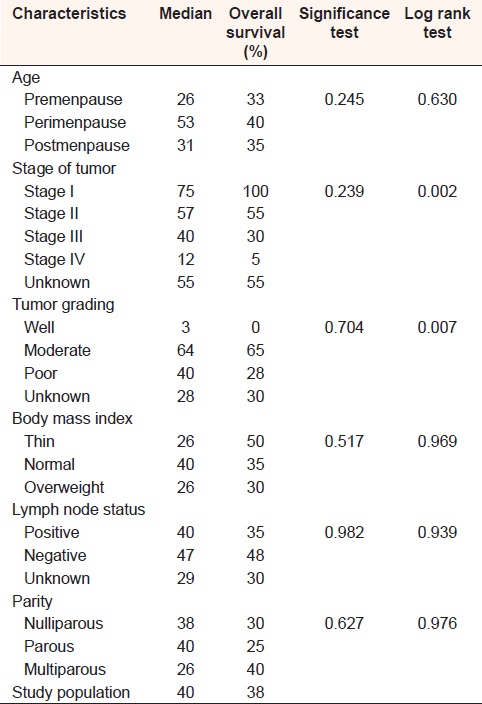 | Table 2: Median survival period, cumulative survival probability, significance and log rank test for the study population according to different characteristics
Click here to view |
| Discussion | |  |
The clinical course of breast cancer is of a more chronic nature than the course of other curable malignancies, such as testicular cancer. Therefore, 5 years and 10 years relapse-free survival is not equivalent to cure. Statistical cure has been defined on the basis of population survival figures. [25],[26],[27] Thus, it is considered that a group of patients with breast cancer has achieved a cure in statistical terms if its survival curve becomes parallel to the survival curve of the general population. For additional precision, these comparisons are made on age-matched populations. The mortality rate of breast cancer increases with clinical and pathological stage. [28],[29] In fact, correlations of clinical characteristics with mortality after treatment defined clinical stages in the first place. [30] Hazard rates of mortality show that there is an initial peak of several years in hazard rates, followed by a gradual decline over subsequent years. [27],[31] The initial peak is higher and narrower for more advanced-stages (stages III and IV); most relapses and deaths occur within the first 3-5 years in these groups. In contrast, patients diagnosed with stages I and II evidence a lower peak that tends to occur later. Thus, the survival curves of patients with more advanced or higher-risk breast cancer start to parallel the survival curves of the general population earlier than the survival curves of the earlier breast cancers. Stated in a different way, although high-risk patients, that is, those with stage III and IV disease are very likely to die from breast cancer, the outcome of those who survive the early years parallel those of the general population, so that this group achieves statistical cure earlier (10-15 years after diagnosis) than lower-risk groups (20-25 years after diagnosis). Because such lengthy follow-up is necessary for complete evaluation of treatment results, some have stated that breast cancer is, in essence, incurable. This argument states that, if follow-up is long enough, relapses will occur. The systematic application of combined modality therapies and mammography screening has shown this position to be mistaken. [32] Clearly, mammographically diagnosed early breast cancer (stages 0 and I) is associated with excellent survival rates, exceeding 90% at 20 years following surgical resection alone or breast conserving therapies. [33] 25 years after diagnosis, the survival curves of patients with stages II, III, and even IV breast cancer suggest relapse-free survival plateau that parallel the survival curves of the general population.
Kaplan-Meier's survival analysis of the current study population indicates that the overall survival probability for the whole study population is 38%, which is a poor rate in comparison with the survival data from developed countries. This could reflect the fact that 41 (64%) patients were initially presented with stages III and IV in which the prognosis is known to be grim. In fact, a total of 78% of Sudanese breast cancer patients have stage III or IV disease (TNM classification) when they first seek medical treatment (data from Sudan FMOH). In these stages, treatment may often involve multiple modalities, including surgery, radiotherapy, chemotherapy, and hormone therapy, and has a markedly diminished chance of success. Therefore, there is an urgent need for better early detection of cancer in Sudan to make treatment more effective, less costly, less invasive, and more accessible and acceptable to patients.
Further analysis according to different variables showed the importance of age, where patients who are more than 40 years of age showed better survival outcome. This data suggests that the disease outcome improves with advancing age. This is evident from reviewing the younger age group which showed the worst outcome of treatment among the study population. This correlates with the traditionally held views that older postmenopausal women with breast cancer have a more indolent course of the disease, by contrast with young patients who have a more aggressive one. However, the extensive literature on this issue is inconsistent, and the cumulative recent data suggests that this particular patient variable is not a very important prognostic factor, particularly when other, more significant; tumor characteristics are taken into account. [34] The tumor grade also showed relevant effect, where moderate differentiated tumors showed better survival than poor or unknown differentiation. Several investigators have also demonstrated in individual series that grade is an important prognostic factor. [34],[35] Lymph nodes involvement also indicated a poor survival in comparison with negative or unknown status of involvement [Table 2]. This could reflect the fact that the most well established prognostic factor in breast cancer is the number of involved axillary lymph nodes harvested, based on at least a level I and level II axillary dissection, together with a detailed histological evaluation of these nodes. [36] An adequate axillary dissection usually contains at least 10 lymph nodes. Recovery of a limited number of lymph nodes at axillary dissection could place the patients incorrectly, according to stage, and lead to inadequate treatment. This may result in an increased regional relapse rate and poorer survival. As the number of involved lymph nodes increases, so does the relapse rate, while the survival rate decreases. [37] Further, more analysis showed also effect of the body mass index on overall survival where patients who were thin or had normal weight at presentation had better outcome [Table 2]. Obesity is associated with increased risk of breast cancer and adverse outcomes in postmenopausal women with the disease. In premenopausal women, however, these associations are less clear. [38],[39],[40]
Chi-square analysis showed that all variables studied were statistically not significant in affecting overall survival, but when log rank test used, stage and tumor grading appeared to have an effect on survival (P = 0.002 and 0.007). These results may reflect the effect of the small study population. Further study with more patients should be conducted before accepting these results.
| Conclusion | |  |
The majority of the patients who presented with advanced-stage disease seem to account for the poor overall survival reported in this study. Early detection of breast cancer by breast self-examination and physician breast examination should be encouraged in Sudan to improve treatment results in breast cancer.
| References | |  |
| 1. | Veronesi U, Boyle P, Goldhirsch A, Orecchia R, Viale G. Breast cancer. Lancet 2005;365:1727-41.  |
| 2. | Parkin DM, Bray F, Ferlay J, Pisani P, Parkin DM, Bray F, et al. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74-108.  |
| 3. | Ferlay J, Bray F, Pisani P, Parkin DM. GLOBOCAN 2002: Cancer Incidence, Mortality and Prevalence Worldwide. Lyon: IARC Press; 2004.  |
| 4. | Vorobiof DA, Sitas F, Vorobiof G, Vorobiof DA, Sitas F, Vorobiof G. Breast cancer incidence in South Africa. J Clin Oncol 2001;19 18 Suppl: 125S-7.  |
| 5. | Omar S, Khaled H, Gaafar R, Zekry AR, Eissa S, el-Khatib O. Breast cancer in Egypt: A review of disease presentation and detection strategies. East Mediterr Health J 2003;9:448-63.  |
| 6. | Adesunkanmi AR, Lawal OO, Adelusola KA, Durosimi MA, Adesunkanmi AR, Lawal OO, et al. The severity, outcome and challenges of breast cancer in Nigeria. Breast 2006;15:399-409.  |
| 7. | Amir H, Kwesigabo G, Aziz MR, Kitinya JN. Breast cancer and conservative surgery in sub Saharan Africa. East Afr Med J 1996;73:83-7.  |
| 8. | Parkin DM, Ferlay J, Hamdi-Cherif M, Sitas F, Thomas Jo, Wabinga H, et al. Cancer in Africa: Epidemiology and Prevention. Lyon: IARC Press; 2003.  |
| 9. | Adesunkanmi AR, Lawal OO, Adelusola KA, Durosimi MA. The severity, outcome and challenges of breast cancer in Nigeria. Breast 2006;15:399-409.  |
| 10. | Gondos A, Brenner H, Wabinga H, Parkin DM. Cancer survival in Kampala, Uganda. Br J Cancer 2005;92:1808-12.  |
| 11. | Ikpatt OF, Kuopio T, Ndoma-Egba R, Collan Y. Breast cancer in Nigeria and Finland: Epidemiological, clinical and histological comparison. Anticancer Res 2002;22:3005-12.  |
| 12. | Morris K. Cancer? In Africa? Lancet Oncol 2003;4:5.  [ PUBMED] |
| 13. | Kerr F, Kerr D. Do we bear any moral responsibility for improving cancer care in Africa? Ann Oncol 2006;17:1730-1.  [ PUBMED] |
| 14. | Parkin DM, Nambooze S, Wabwire-Mangen F, Wabinga HR. Changing cancer incidence in Kampala, Uganda, 1991-2006. Int J Cancer 2010;126:1187-95.  |
| 15. | Fregene A, Newman LA. Breast cancer in sub-Saharan Africa: How does it relate to breast cancer in African-American women? Cancer 2005;103:1540-50.  |
| 16. | Hamad HM. Cancer initiatives in Sudan. Ann Oncol 2006;17 Suppl 8:viii32-6.  |
| 17. | Ahmed HG, Ali AS, Almobarak AO. Frequency of breast cancer among Sudanese patients with breast palpable lumps. Indian J Cancer 2010;47:23-6.  [ PUBMED]  |
| 18. | Awadelkarim KD, Arizzi C, Elamin EO, Hamad HM, De Blasio P, Mekki SO, et al. Pathological, clinical and prognostic characteristics of breast cancer in Central Sudan versus Northern Italy: Implications for breast cancer in Africa. Histopathology 2008;52:445-56.  |
| 19. | Awadelkarim KD, Aceto G, Veschi S, Elhaj A, Morgano A, Mohamedani AA, et al. BRCA1 and BRCA2 status in a Central Sudanese series of breast cancer patients: Interactions with genetic, ethnic and reproductive factors. Breast Cancer Res Treat 2007;102:189-99.  |
| 20. | Awadelkarim KD, Mohamedani AA, Barberis M. Role of pathology in sub-Saharan Africa: An example from Sudan. Pathol Lab Med Int 2010;2:49-57.  |
| 21. | INMO Annual Report. INMO Annual Report. Report. Wad Medani: Information and Research Center, Statistic Unit, Department of Oncology, Institute of Nuclear Medicine Molecular Biology and Oncology (INMO), Wad Medani, Sudan; 2006.  |
| 22. | INMO Annual Report. INMO Annual Report. Report. Wad Medani: Information and Research Center, Statistic Unit, Department of Oncology, Institute of Nuclear Medicine Molecular Biology and Oncology (INMO), Wad Medani, Sudan; 2001.  |
| 23. | Parkin DM, Muir CS. Cancer Incidence in Five Continents. Comparability and quality of data. IARC Sci Publ 1992;6:45-173.  |
| 24. | Kaplan EL, Meier P. Nonparametric estimation from incomplete observaton. J Am Stat Assoc 1958;53:547-81.  |
| 25. | Hortobagyi GN. The curability of breast cancer: Present and future. EJC Suppl 2003;1:24-34.  |
| 26. | Woods LM, Rachet B, Cooper N, Coleman MP. Predicted trends in long-term breast cancer survival in England and Wales. Br J Cancer 2007;96:1135-8.  |
| 27. | Clare SE, Nakhlis F, Panetta JC. Molecular biology of breast cancer metastasis. The use of mathematical models to determine relapse and to predict response to chemotherapy in breast cancer. Breast Cancer Res 2000;2:430-5.  |
| 28. | Nemoto T, Vana J, Bedwani RN, Baker HW, McGregor FH, Murphy GP. Management and survival of female breast cancer: Results of a national survey by the American College of Surgeons. Cancer 1980;45:2917-24.  [ PUBMED] |
| 29. | Rutqvist LE, Wallgren A, Nilsson B. Is breast cancer a curable disease? A study of 14,731 women with breast cancer from the Cancer Registry of Norway. Cancer 1984;53:1793-800.  [ PUBMED] |
| 30. | Yarbro JW, Page DL, Fielding LP, Partridge EE, Murphy GP. American Joint Committee on Cancer prognostic factors consensus conference. Cancer 1999;86:2436-46.  |
| 31. | Kerr GR, Kunkler IH, Langlands AO, Rodger A. (In) curability of breast cancer: A 30-year report of a series of 3933 cases. Breast 1998;7:90-4.  |
| 32. | Tabár L, Vitak B, Chen HH, Duffy SW, Yen MF, Chiang CF, et al. The Swedish Two-County Trial twenty years later. Updated mortality results and new insights from long-term follow-up. Radiol Clin North Am 2000;38:625-51.  |
| 33. | Sun J, Chapman J, Gordon R, Sivaramakrishna R, Link M, Fish E. Survival from primary breast cancer after routine clinical use of mammography. Breast J 2002;8:199-208.  |
| 34. | Lee CG, McCormick B, Mazumdar M, Vetto J, Borgen PI. Infiltrating breast carcinoma in patients age 30 years and younger: Long term outcome for life, relapse, and second primary tumors. Int J Radiat Oncol Biol Phys 1992;23:969-75.  |
| 35. | Gajdos C, Tartter PI, Bleiweiss IJ, Bodian C, Brower ST. Stage 0 to stage III breast cancer in young women. J Am Coll Surg 2000;190:523-9.  |
| 36. | Harris JR, Morrow M, Norton N. Malignant tumors of the breast. Cancer: Principal and Practice of Oncology. 5 th ed., Sec. 2, Ch. 36. Philadelphia, PA, USA: Lippincott and Raven Publishers; 1997. p. 1590-1.  |
| 37. | Weir L, Speers C, D'yachkova Y, Olivotto IA. Prognostic significance of the number of axillary lymph nodes removed in patients with node-negative breast cancer. J Clin Oncol 2002;20:1793-9.  |
| 38. | Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 2003;348:1625-38.  |
| 39. | Petrelli JM, Calle EE, Rodriguez C, Thun MJ. Body mass index, height, and postmenopausal breast cancer mortality in a prospective cohort of US women. Cancer Causes Control 2002;13:325-32.  |
| 40. | Morimoto LM, White E, Chen Z, Chlebowski RT, Hays J, Kuller L, et al. Obesity, body size, and risk of postmenopausal breast cancer: The Women's Health Initiative (United States). Cancer Causes Control 2002;13:741-51.  |
[Figure 1]
[Table 1], [Table 2]
|