|
 
 |
| ORIGINAL ARTICLE |
|
| Year : 2016 | Volume
: 11
| Issue : 1 | Page : 1-6 |
|
Umbilical cord abnormalities
Elghazaly Abdulrahim Elghazaly1, Qurashi M Ali2, Haydar Hadi Babikir3
1 Department of Human Anatomy, Faculty of Medicine and Health Sciences, Omdurman Islamic University, Omdurman, Sudan
2 Department of Anatomy, National University, Khartoum, Sudan
3 Department of Pediatric, University of Gezira, Wad Madani, Omdurman, Sudan
| Date of Web Publication | 10-Mar-2016 |
Correspondence Address:
Elghazaly Abdulrahim Elghazaly
Department of Human Anatomy, Faculty of Medicine and Health Sciences, Omdurman Islamic University, Omdurman
Sudan
  | Check |
DOI: 10.4103/1858-5000.178489 
Objective: Stillbirths and neonatal outcome after delivery has been associated with umbilical cord findings. Cord abnormalities may put the baby in higher risk of certain diseases or low birth weight. The etiology of the umbilical cord abnormalities are not clear diagnosis. Although the fetal mortality associated with umbilical cord abnormalities is very high, identification of the fetus at risk has remained a difficult problem. The aim of this academic work is to study the human umbilical cord (UC) congenital abnormalities including length, diameter, coiling pattern, and placental insertion site in full term Sudanese neonates. Materials and Methods: One thousand and twenty UC of full-term birth neonates of both sexes, single and twin, of normal vaginal delivery in 2013, were studied in the Department of Obstetrics and Gynecology, Omdurman Maternity Hospital. UC length was entirely measured in centimeters using flexible plastic meter and the diameter was measured by Vernier caliper. The number of UC coils, coils pattern, insertion site, knots, cysts, and the blood vessels were fully studied. Results: The average UC length was about 58 cm, with minimal difference between male to female, single to twin birth. In majority of the cases, true knots occur without any clinical significance, whereas the tight knot may impede the circulation and may lead to low birth or intrauterine death. Thin cord places fetus at risk during pregnancy and may result in restricted growth, low birth weight, and neonates which are small for gestational age. This appears to be a consequence of reduction in the area of Wharton's jelly or small cord diameter. Very helical designs may predispose the fetus to certain blood flow changes, and very straight designs may be susceptible to compression. In both these types of coils, the degree of coils vary depending on cord length, diameter, fetal gender, and number of pregnancy. Hypercoiled coils may predispose the fetus to certain blood flow changes and hypocoiled cords may lead to the fetus being susceptible to compression; however in both cases, the blood flow and fetal weight may be affected. These factors were considered regarding possible interactions and correlation to pregnancy and perinatal outcome. Conclusion: Average length of UC in Sudanese neonates about 55 cm, being long in males and single birth, and there is no big difference in the cord length measurement before and after birth, comparing the two methods (ultrasound and manual measurements). 20cm of the cord length is suitable for fetus to be delivered vaginally. Average UC diameter in Sudanese neonates about 1.5 cm, big in males and single birth, Maximum UC coils about 45 coils, more in males and single birth, eccentric insertion is more common than central or marginal false knots of the are also more common than true knots. Most of the cord true knots located near to the fetal end of the umbilical cord, and they were commonly coiled to the left side (anticlockwise direction) incidence of a SUA in Sudanese neonate about 1% more common in males Keywords: Abnormalities, Sudanese, umbilical cord
How to cite this article:
Elghazaly EA, Ali QM, Babikir HH. Umbilical cord abnormalities. Sudan Med Monit 2016;11:1-6 |
| Introduction | |  |
Throughout human history, stillbirths have been associated with umbilical cord (UC) findings. The congenital abnormalities of the UC have been reported as early as 1925. [1],[2] The abnormalities vary, and the variations of UC complications exist depending on factors such as the time and degree of compression. [3] The etiology of the UC abnormalities is not diagnosed clearly. UC abnormalities can be detected before birth by ultrasound and after birth following examination of the cord and placenta. Although the fetal mortality associated with UC abnormalities is very high, identification of the fetus at risk has remained difficult. These abnormal UC are predisposed to rupture, mechanical failure, entanglement, disruption of labor, uterine malfunction, and premature labor. The ultimate effect is the disturbance of the life line and derangement of blood flow to the fetus. The difficulty lies in the fact that these abnormalities are silent and invisible. The following are the most frequent UC abnormalities and their possible effects on mother and baby.
| Materials and Methods | |  |
UC length, diameter, coiling pattern, placental insertion site, and blood vessels was studied in 1020 full-term birth neonates of both sexes, single and twin, of normal vaginal delivery, between February and September 2013, in the Department of Obstetrics and Gynecology, Omdurman Maternity Hospital. Still birth, elective cesarean section, and abnormal vaginal deliveries were excluded. All subjects included in this study looked healthy. The umbilical cord cut about 5 centimeters from the baby abdominal wall (fetal part) by scissor. The rest of the cord from the cut end to the placental insertion (placental part) is measure in centimeters, using flexible plastic meter. The five centimeters added to the rest of the cord. UC coils were counted and their direction was observed and identified. Coil design appeared different, so they are classified into three types based on coil pattern. Type I, the umbilical vessels (UV) were course in a parallel fashion. Type II, the UV were run in coiling course. Type II was classified into A and B and in type II A, umbilical vein is predominantly straight and umbilical arteries coiled around umbilical vein and in type II B, the UV course together in a helical fashion. Type III, is tortuous or spiral cord, in which the cord coils upon itself. The cord diameter was measured using Vernier caliper. The fetal surface of the placenta was observed to identify the cord insertion, whether central, eccentric, or marginal. UC knots, cysts, and blood vessels also were observed and studied from different angles. Data were entered to computer software programs and analyzed using Statistical Package for Social Science version 16 (SPSS, V16. Aromonk, NY: IBM Corp, USA).
| Results | |  |
Out of all UC specimens, 584 (57.3%) were male cords and 436 (42.7%) were female cords [Figure 1], and 980 (98%) cords of single birth and 20 (2%) were of twin birth babies [Figure 2].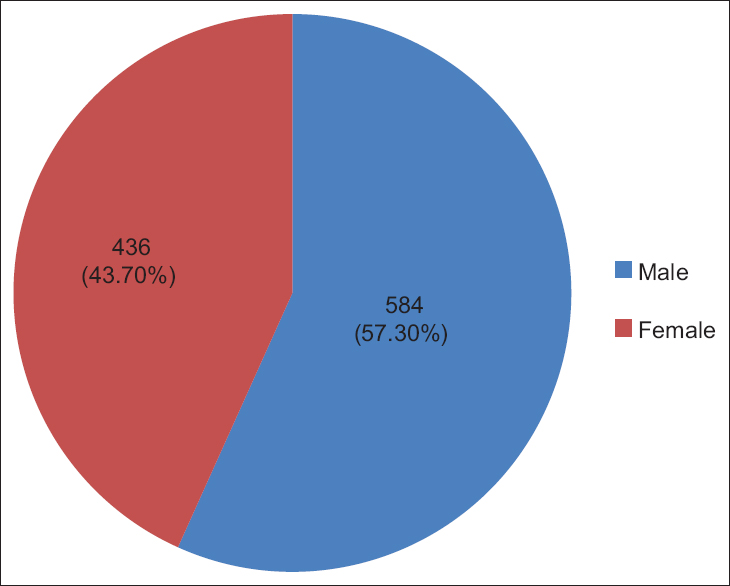 | Figure 1: Sex outcome, of the 1020 Sudanese neonates, in the Department of Obstetrics and Gynecology, Omdurman Maternity Hospital, 2013
Click here to view |
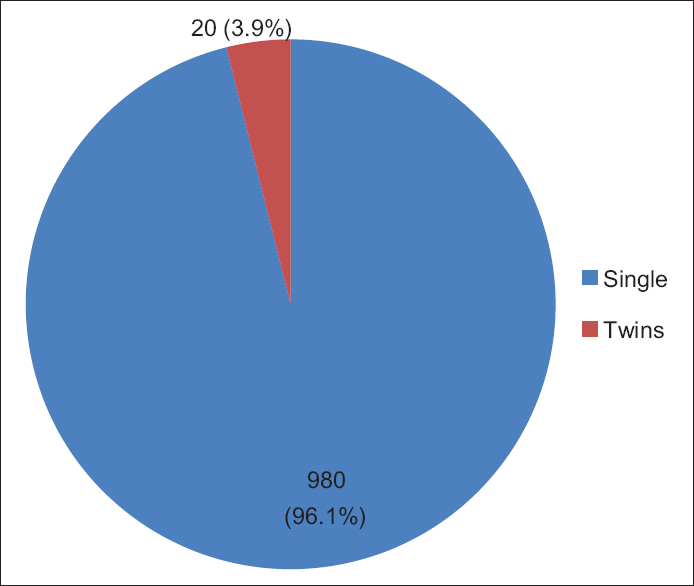 | Figure 2: Birth outcome, of the 1020 Sudanese neonates, in the Department of Obstetrics and Gynecology, Omdurman Maternity Hospital, 2013
Click here to view |
Grossly the attachment of UC to the fetal placental surface was observed, which is shown in [Figure 3]. Eccentric insertion in which the cord inserted near the center of the placenta was about 850 (83.3%). Central insertion in which the cord inserted to the center of the placenta was about 134 (13.2%). Marginal insertion in which the cord inserted on the placental edge was about 36 (3.5%).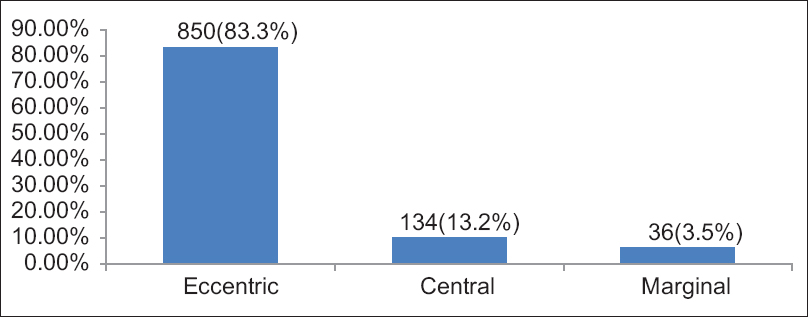 | Figure 3: Types of the umbilical cord insertion, in 1020 Sudanese neonates, Department of Obstetrics and Gynecology, Omdurman Maternity Hospital, 2013
Click here to view |
The UC knot appeared in 126 (12.4%) specimens. Out of these, 114 (11.2%) were false knots and 12 (1.2%) were true knots [Figure 4]. Out of 12 true knots, 8 (66.7%) were occurred near to the fetal of the UC 3 (25%) at the middle, and 1 (8.3%) near the placental end of the UC, all of which appeared single [Figure 5]. Out of the true knots, 8 (66.7%) were coiled to the left (anticlockwise direction) and 4 (33.3%) were coiled to the right (clockwise direction) [Figure 6]. The ratio of the left to right true knot coiling direction was approximately 2:1. The false knots appeared of variable size, numbers, location, and shape. The UC cysts were varied and showed in 42 (4.1%) specimens out of 1020 cords sample [Table 1].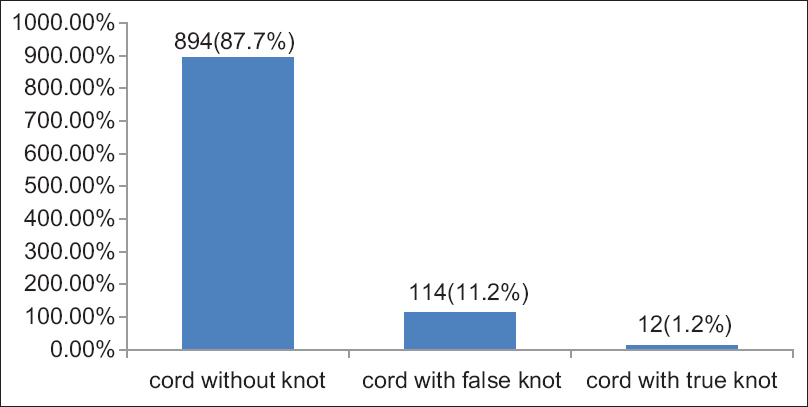 | Figure 4: Umbilical cord knot, in 1020 Sudanese neonates, in the Department of Obstetrics and Gynecology, Omdurman Maternity Hospital, 2013
Click here to view |
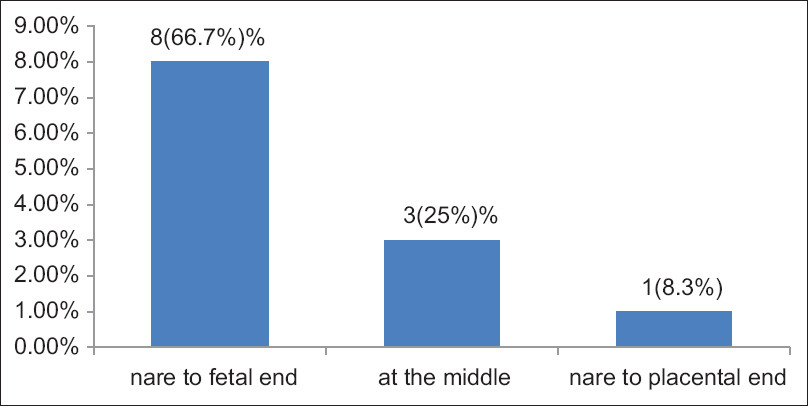 | Figure 5: Site of the umbilical cord true knot, in 1020 Sudanese neonates, in the Department of Obstetrics and Gynecology, Omdurman Maternity Hospital, 2013
Click here to view |
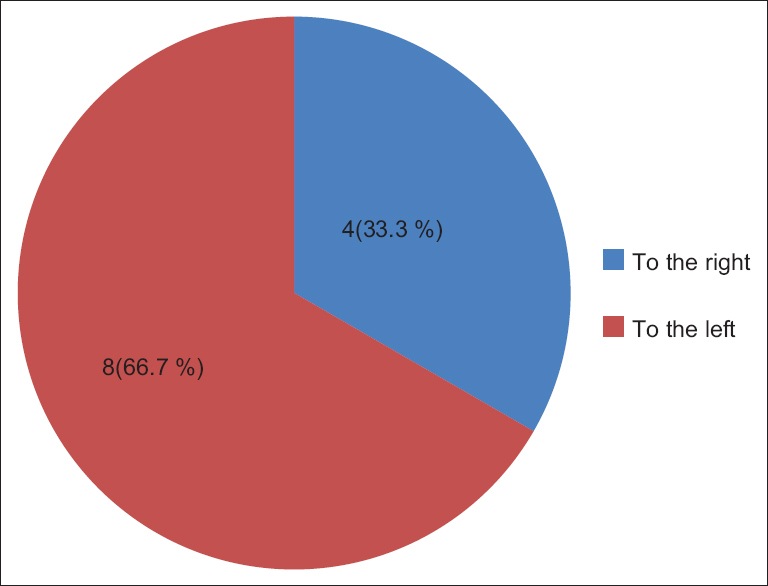 | Figure 6: Umbilical cord true knot coiling direction, in 1020 Sudanese neonates birth, in the Department of Obstetrics and Gynecology, Omdurman Maternity Hospital, 2013
Click here to view |
 | Table 1: Umbilical cord cyst in Sudanese neonates, in the Department of Obstetrics and Gynecology, Omdurman Maternity Hospital, 2013
Click here to view |
The number of UC coils in single birth babies ranged from 4 to 45 coils with mean (13.2222 ± 7.96597 standard deviation [SD]) in male and from 4 to 36 coils with mean (12.2358 ± 7.47579 SD) in female. In the twin birth babies, the coils ranged from 6 to 18 coils with mean (13.0000 ± 4.89898 SD) in male and from 6 to 32 coils with mean (15.1668 ± 10.13395 SD) in female [Table 2]. Out of all, the UCs specimens 759 (74.4%) were coiled off to left (anticlockwise direction) and 261 (25.6%) were coiled to right (clockwise direction) from placental end of the cord [Figure 7]. The outcomes ratio of the left to right twisting approximately 3:1. On the basis of coiling number, the cords were grouped as hypocoiled group which have <10 coils, normocoiled group have coils between 10 and 40, and hypercoiled have more than 40 coils [Table 3].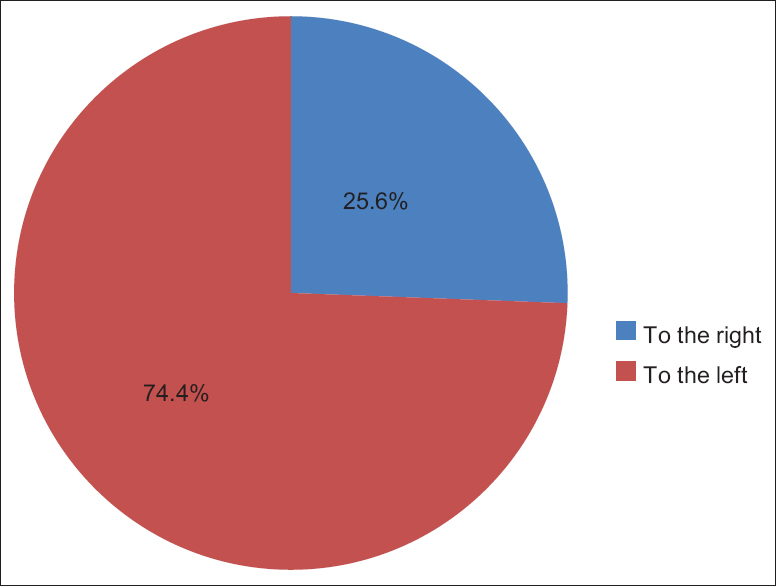 | Figure 7: Direction of the umbilical cord coils, among 1020 Sudanese neonates birth, in the Department of Obstetrics and Gynecology, Omdurman Maternity Hospital, 2013
Click here to view |
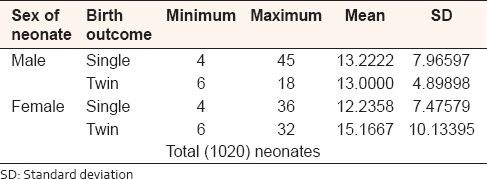 | Table 2: Number of the umbilical cord coils in single and twin, Sudanese birth, in the Department of Obstetrics and Gynecology, Omdurman Maternity Hospital, 2013
Click here to view |
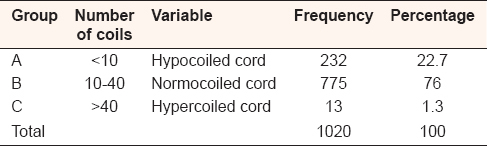 | Table 3: Umbilical cord coil number in groups, in single and twin Sudanese birth, in Omdurman Maternity Hospital of Obstetrics and Gynecology, 2013
Click here to view |
The length of the UC in single birth babies ranged from 24 to 90 cm with mean (47.4167 ± 11.48761 SD) in male, and from 20 to 86 cm with mean (44.7547 ± 11.29347 SD) in female, and in twin birth babies, the cord length ranged from 32 to 42 cm with mean (35.6500 ± 4.02670 SD) in male, and ranged from 30 to 48 cm with mean (36.6667 ± 6.78680 SD) in female [Table 4].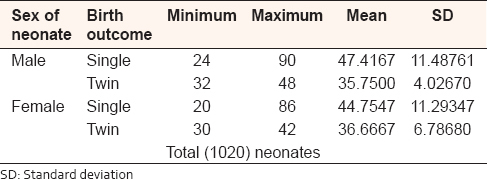 | Table 4: Umbilical cord length, in centimeter of the single and twin Sudanese birth, in the Department of Obstetrics and Gynecology, Omdurman Maternity Hospital, 2013
Click here to view |
The diameter of the UC in single birth babies ranged from 0.5 to 2.5 cm with mean (1.1582 ± 24093 SD) in male, and ranged from 0.5 to 1.6 cm with mean (1.0939 ± 16011 SD) in female. In twin birth babies, the cord diameter ranged from 0.8 to 1.2 cm with mean (0.9 ± 0.18516 SD) in male and ranged from 0.7 to 1.2 cm with mean (0.9 ± 0.17056 SD) in the female [Table 5].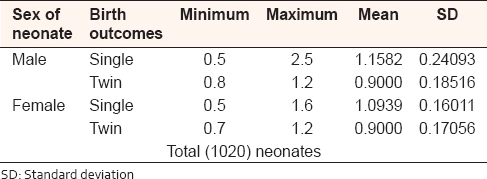 | Table 5: Umbilical cord diameter, in single and twin Sudanese birth, in the Department of Obstetrics and Gynecology, Omdurman Maternity Hospital, 2013
Click here to view |
Out of 1020 UC specimens, 110 (99%) cords had three blood vessels, two arteries, and one vein, and 10 (1.0%) had two blood vessels, one artery, one vein, and single umbilical artery (SUA) [Figure 8]. The SUA was found in single birth babies; out of them, 6 were male and 4 female, the outcome ratio of male-to-female with SUA is 3:2.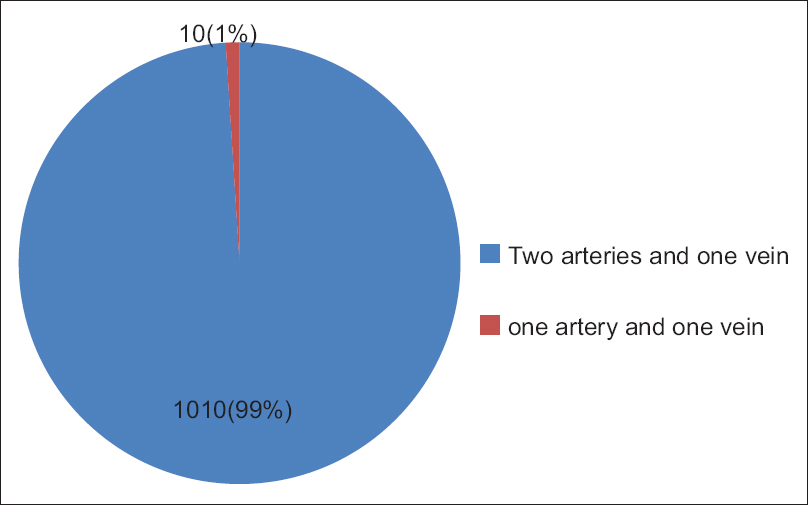 | Figure 8: Number of umbilical cord blood vessels, in 1020 Sudanese neonates, in the Department of Obstetrics and Gynecology, Omdurman Maternity Hospital, 2013
Click here to view |
| Discussion | |  |
Several studies were done to measure human UC length using various factors. [4],[5],[6] In most of them, the length ranged from 60 to 70 cm with an average of 50 cm. The average UC length in this study was 58 cm, with small difference between male to female and single to twin birth, as shown in [Table 4]. This result was nearly the results of previous studies. Hence, there is no big difference in the cord length measurement before and after the birth; the difference may refer to differences in their birth weight or height. In 1958 Javert [7] found that the length of the UC was usually the same as the standing height of the fetus at all stages of pregnancy. Javert defined a short cord as one less than one-third the standing height of the fetus and long cord one as three or more times his height. According to Malpas, [8] a British obstetrician who studied cord length in the 1960s, he reported that the average cord length was about 61 cm, but Collins in 2014 [9],[10] reported that cord length had a positive correlation with male gender. However, very long cord in spite of giving freedom and fetal mobility is sometimes liable to more coils, entanglement, true knots, and vessel compression. Today, cord length correlates to several outcomes such as fetal position, activities, growth, uterine size, neonatal gender, neurologic abnormalities, and it is predisposes the fetus to intrauterine dangers. Incidence of true knot of the UC is not only very low, but it is often diagnosed antenatally or postnatally, but postnatal is the most common. In majority of the cases, true knots occur without any clinical significance, but the tight knot may impede the circulation and may lead to intrauterine death. True knots correlate with the cord length of low birth weight babies. In Hertzberg et al 1988, [10] described that true knot can occur anywhere through cord length. Furthermore the present study found that most of the true knots were occurring near to the fetal end of the umbilical cord, and coiled to the left side (anticlockwise direction), because this part moves with fetus. This could explain why the fetus rotates mainly on the left side of uterus, even during pregnancy examination most fetal back are commonly found located on the left side of the uterus. The false knot has no known clinical significance, as mentioned by Edmonds, [11] but if it becomes multi-knots it may interrupt blood flow, and reduce fetal growth and birth weight.
Thin cord place fetuses at risk during pregnancy with restricted growth and birth weight, classified as small for gestational age. This appears to be a consequence of a reduction in the area of Wharton's jelly (WJ) or small cord diameter. The cord diameter depends on the amount of WJ. Cord diameter in this present study was found to not exceed 2.5 cm, but if it exceeds 2.5 cm; it is termed abnormal cord and is associated with edema, tumor, or multiple cysts.
Helical or twisting appearance of the UC is a likely to be due to the movement of the fetus within the uterus or helical course of the UC vessels. Because the arteries are longer than the vein, arteries develop tortuosity, and tortuosity increases readily because they lie free in viscoelastic sheath of the WJ without branching and restricted anatomical structures or neighboring organs. Very helical designs may predispose the fetus to certain blood flow changes, and very straight designs may be susceptible to compression. [12],[13] Edmonds [11] in (1954) and Malpas and Symonds [8] in (1966), they studied the characteristic of the cord coils and reported that the maximum cord coils should be about 8-11, Bourke et al. [12] in 1993, described 308 coils, the present study found 45 coils. The difference may refer to the differences in time and the type of technique. More coils as described by Bourke et al. [12] in 1993 may predispose the fetus to certain blood flow changes. Any cord shows coils, but the degree of coils vary depending on the cord length, diameter, fetal gender, and number of pregnancies. Hypercoiled coils may predispose the fetus to certain blood flow changes and hypocoiled cords make them susceptible to compression; however in both cases, the bloods flow and fetal weight may be affected. The vein is more susceptible to compression, torsion, and kinking than arteries. The UC was found with commonly eccentric insertion; this was in agreement with most of the previous studies. Abnormal cord insertion gives loose connection of cord with placenta, this increases the risk of cord vessels rupture in which the blood flow will reduce and decrease fetal growth. The abnormal UC insertion was most frequently found in monozygotic twins; these factors were considered regarding possible interactions and correlation to pregnancy and perinatal outcome.
Normally the UC has three vessels, absence of one umbilical artery, defined as a SUA. The exact cause of SUA is unknown, but Ghatersamni et al. [13] in (2007) suggested that it occurs due to primary agenesis or secondary atrophy of one artery in the early stages of embryonic life. In addition, in 1991 Nyberg et al., [14] reported that the atrophy is probably the most frequent mechanism. Incidence of SUA has been reported in most of the previous studies, it occurs in 1% of deliveries, and are more common in female than in male babies by as described by Nyberg et al. 1991 The same result was found in our study. A number of studies have reported that the presence of SUA may be related to a variety of congenital anomalies of the major organ systems as well as to chromosomal defects and low birth weight. [15],[16],[17],[18] There was no specific pattern of associated anomalies in the present study, but there was predominance in two cases of nervous system abnormalities, spina bifida associated with hydrocephalus. Four vessel UCs are rare and has been reported by Heifetz [18] in conjoined twins. Cord with four blood vessels were not found in the present study, either in single or twin birth.
| Conclusions | |  |
- The average length of the umbilical cord in Sudanese neonates about 55 cm, being long in males and single birth. There is no big difference in the cord length measurement before and after birth, comparing the two methods (ultrasound and manual measurements). 20cm of the cord length is suitable for fetus to be delivered vaginally
- Average cord diameter in Sudanese neonates about 1.5 cm, being big in males and single birth
- Maximum umbilical cord coils in Sudanese neonates about 45 coils, being, more in males and single birth
- In Sudanese neonates the eccentric umbilical cord insertion to placenta is more common than central and marginal
- The false knots of the umbilical cord are more common than true knots. False knot occurs due to increased length of the umbilical vein in comparison to arteries, and has no known clinical significance
- Most of the cord true knots located near to the fetal end of the umbilical cord, and they were commonly coiled to the left side (anticlockwise direction)
- The incidence of a SUA in Sudanese neonate about 1% more common in males.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| References | |  |
| 1. | Browne FJ. The abnormalities of the umbilical cord which may cause antenatal death. J Obstet Gynaecol Br Emp 1925;32:17-20.  |
| 2. | Pathak S, Hook E, Hackett G, Murdoch E, Sebire NJ, Jessop F, et al. Cord coiling, umbilical cord insertion and placental shape in an unselected cohort delivering at term: Relationship with common obstetric outcomes. Placenta 2010;31:963-8.  |
| 3. | Sepulveda W, Sebire NJ, Harris R, Nyberg DA. The placenta, umbilical cord, and membranes. In: Nyberg DA, McGahan JP, Pretorius DH, Pilu G, editors. Diagnostic Imaging of Fetal Anomalies. Philadelphia, PA: Lippincott Williams and Wilkins; 2003. p. 85-132.  |
| 4. | Beall MH, Ross MG. Umbilical cord complications. J Obstet Gynecol 2009;24:218-23.  |
| 5. | Balkawade NU, Shinde MA. Study of length of umbilical cord and fetal outcome: A study of 1,000 deliveries. J Obstet Gynaecol India 2012;62:520-5.  |
| 6. | Katsumata T, Miyake A, Aki T, Hirooka K, Hayashida M, Toyoda M, et al. Length of the human umbilical cord in multiple pregnancy. Eur J Obstet Gynecol Reprod Biol 1991;40:25-7.  |
| 7. | Javert CT. Prevention of habitual spontaneous abortion with early prenatal care. Bull N Y Acad Med 1958;34:747-56.  [ PUBMED] |
| 8. | Collins JH. Umbilical cord accidents and legal implications. Semin Fetal Neonatal Med 2014;19:285-9.  |
| 9. | Sørnes T. Umbilical cord knots. Acta Obstet Gynecol Scand 2000;79:157-9.  |
| 10. | Hertzberg BS, Bowie JD, Bradford WD, Bolick D. False knot of the umbilical cord: Sonographic appearance and differential diagnosis. J Clin Ultrasound 1988;16:599-602.  |
| 11. | Edmonds HW. The spiral twist of the normal umbilical cord in twins and in singletons. Am J Obstet Gynecol 1954;67:102-20.  [ PUBMED] |
| 12. | Malpas P, Symonds EM. The direction of the helix of the human umbilical cord. Ann Hum Genet 1966;29:409-10.  [ PUBMED] |
| 13. | De Laat MW, van der Meij JJ, Visser GH, Franx A, Nikkels PG. Hypercoiling of the umbilical cord and placental maturation defect: Associated pathology? Pediatr Dev Pathol 2007;10:293-9.  |
| 14. | Bourke WG, Clarke TA, Mathews TG, O'Halpin D, Donoghue VB. Isolated single umbilical artery - The case for routine renal screening. Arch Dis Child 1993;68:600-1.  |
| 15. | Ghatersamni F, Javadrashid R, Tarzamni MK. Single umbilical artery: Prevalence and clinical significance in prenatal sonography. J Obstet Gynecol Radiol 2007;17:269-72.  |
| 16. | Nyberg DA, Mahony BS, Luthy D, Kapur R. Single umbilical artery. Prenatal detection of concurrent anomalies. J Ultrasound Med 1991;10:247-53.  |
| 17. | Gornall AS, Kurinczuk JJ, Konje JC. Antenatal detection of a single umbilical artery: Does it matter? Prenat Diagn 2003;23:117-23.  |
| 18. | Mu SC, Lin CH, Chen YL, Sung TC, Bai CH, Jow GM. The perinatal outcomes of asymptomatic isolated single umbilical artery in full-term neonates. Pediatr Neonatol 2008;49:230-3.  |
[Figure 1], [Figure 2], [Figure 3], [Figure 4], [Figure 5], [Figure 6], [Figure 7], [Figure 8]
[Table 1], [Table 2], [Table 3], [Table 4], [Table 5]
|