|
 
 |
| ORIGINAL ARTICLE |
|
| Year : 2017 | Volume
: 12
| Issue : 1 | Page : 13-17 |
|
Association of subclinical hypothyroidism and lipid profile in dyslipidemic patients from Gaza City, Palestine
Mahmoud I El-Habiby1, Loay Naser2, M Morar1, Aia Almasry1, Marghoob Hasan3, Abdelmarouf Hassan Mohieldein3
1 Department of Medical Laboratory Sciences, Al-Aqsa University, Al-Qassim, Kingdom of Saudi Arabia
2 Medical Services, Gaza, Palestine, Al-Qassim, Kingdom of Saudi Arabia
3 Department of Medical Laboratories, College of Applied Medical Sciences, Qassim University, Al-Qassim, Kingdom of Saudi Arabia
| Date of Web Publication | 18-May-2017 |
Correspondence Address:
Abdelmarouf Hassan Mohieldein
Department of Medical Laboratories, College of Applied Medical Sciences, Qassim University, P.O.Box: 6699, Buraidah 51452, Al-Qassim,
Kingdom of Saudi Arabia
  | Check |
DOI: 10.4103/summ.summ_37_16 
Background: Dyslipidemia covers the broad spectrum of lipid abnormalities. Subclinical hypothyroidism (SCH) is a clinical status of mildly elevated serum thyroid-stimulating hormone (TSH) levels with normal levels of free T4 and free T3. Aims: The aim of this study was to examine the impact of SCH in patients with dyslipidemia in Gaza City, Palestine. We also aimed to investigate the associations between TSH and lipid profiles. Settings and Design: In a case–control study, a total of eighty individuals are involved in the study, of them forty were patients with dyslipidemia and forty were healthy individuals who represented the control group. Patients were examined and diagnosed by a physician in the dyslipidemic clinic, Palestinian Medical Relief Society in Gaza Strip. Materials and Methods: Blood sample collected in plain tubes for the preparation of serum. The serum TSH, total cholesterol (TC), high-density lipoprotein (HDL), and triglycerides levels were measured using standardized assays. A prepiloted questionnaire was used to collect the demographic data and information about family history of dyslipidemia and remove fat from meat. Statistical Analysis Used: Data analyses were performed using the Statistical Package for the Social Sciences (SPSS) software (version 17). Continuous data were expressed as a mean ± standard deviation, whereas categorical data as number (%). Odd ratios were calculated using Win Episcope to find the association between selected variables and dyslipidemia associated with SCH.P < 0.05 was considered statistically significant. Results: Patients with dyslipidemia manifested significant higher TSH compared to controls (P = 0.049), whereas triiodothyronine and tetraiodothyronine levels were not showed significant differences between cases and controls. Moreover, TSH level was positively correlated with TC (r = 0.445,P = 0.000) and low-density lipoprotein-cholesterol (r = 0.415,P = 0.000) but negatively to HDL-cholesterol (r= -0.422 andP = 0.000). Logistic regression analysis showed that female, smoking, and positive history of dyslipidemia were risk factors for SCH in study participants. Conclusions: Findings from the present study reflect the association of dyslipidemia and SCH. The patients in case group showed significant lipids' alteration and higher TSH when compared to controls. Moreover, this study reported a strong relationship between dyslipidemia and clinical hypothyroidism. Keywords: Dyslipidemia, Gaza City, lipid profile, Palestine, subclinical hypothyroidism, thyroid-stimulating hormone
How to cite this article:
El-Habiby MI, Naser L, Morar M, Almasry A, Hasan M, Mohieldein AH. Association of subclinical hypothyroidism and lipid profile in dyslipidemic patients from Gaza City, Palestine. Sudan Med Monit 2017;12:13-7 |
How to cite this URL:
El-Habiby MI, Naser L, Morar M, Almasry A, Hasan M, Mohieldein AH. Association of subclinical hypothyroidism and lipid profile in dyslipidemic patients from Gaza City, Palestine. Sudan Med Monit [serial online] 2017 [cited 2018 Jul 22];12:13-7. Available from: http://www.sudanmedicalmonitor.org/text.asp?2017/12/1/13/206562 |
| Introduction | |  |
Dyslipidemia covers the broad spectrum of lipid abnormalities.[1] Dyslipidemia includes high blood levels of total cholesterol (TC), low-density lipoprotein-cholesterol (LDL-C), triglycerides (TG), and low levels of high-density lipoprotein-cholesterol (HDL-C).[2],[3]
The main hormones of thyroid gland are triiodothyronine (T3) and tetraiodothyronine (T4, thyroxine) which regulate the growth rate and function of many other systems in the body.[4] With specific regard to lipid metabolism, thyroid hormones play an important role in synthesis, mobilization, and metabolism of lipids, but degradation is affected more than synthesis.[5],[6]
The term subclinical denotes the presence of a disease without obvious symptoms, which means that evolution of the disease might be at an early stage.[7] Subclinical hypothyroidism (SCH) is a clinical status of mildly elevated serum thyroid-stimulating hormone (TSH) levels (up to 10 mU/L) with normal levels of free T4 (FT4) and free T3 (FT3) and is far more common and asymptomatic disorder than overt hypothyroidism.[8] The prevalence of SCH in the general population ranges from 1% to 10% and reaches 16% in elderly women.[9]
This is the first study conducted to assess the impact of SCH on dyslipidemic patients in Gaza City, Palestine. Our aim was to evaluate the effect of SCH on dyslipidemic patients. Furthermore, we interested to investigate the associations between TSH and lipid profiles. The outcomes of this study may provide evidence and recommendations on current information about dyslipidemia and SCH which may contribute to improve the health status of patients.
| Materials and Methods | |  |
Study design and subjects
This case–control study was conducted in Biochemistry Laboratory, Department of Medical Laboratory Sciences, Al-Aqsa University, Gaza, Palestine. Forty dyslipidemic patients (aged 40–60 years) recruited as cases. They were examined and diagnosed by a physician in the dyslipidemic clinic, Palestinian Medical Relief Society in Gaza Strip. In addition, another forty age-matched healthy participants invited and included in the control group.
Blood sampling
For each participant, 5 ml of blood was collected by venipuncture in a plain vacutainer for serum preparation to determine lipid profile (TC, TG, and HDL-C) and TSH. The collected blood samples were allowed to clot at room temperature, and serum was separated by centrifugation at 3000 RPM for 10 min and stored at -20°C until analysis. A questionnaire was used to collect the demographic data about participants.
Determination of lipids profile and definition of dyslipidemia
Serum TC and TG were measured by Trinder reaction, whereas HDL-C was determined by polyanion precipitation method. The commercial kits for lipid profile measurement were purchased from DiaSys Diagnostic Systems, Holzhein, Germany. Serum LDL-C was calculated by the Friedewald formula LDL-C = TC - HDL-C - TG/5 (mg/dL), whenever TG >400 mg/dL.
Dyslipidemia was defined as per the National Cholesterol Education Program Adult Treatment Panel III guidelines: TC ≥200 mg/dL; LDL-C ≥100 mg/dL; TG level ≥150 mg/dL; and HDL-C ≤40 mg/dL.[10]
Determination of thyroid-stimulating hormone
Serum TSH was measured using Ultrasensitive-TSH ELISA using DRG ® TSH ELISA (EIA-4171) kit purchased from DRG International, Inc., USA.
The assay system utilizes a unique monoclonal antibody directed against a distinct antigenic determinant on the intact TSH molecule. Mouse monoclonal anti-TSH antibody was used for solid phase (microtiter wells) immobilization, and goat anti-TSH antibody was used in the antibody-enzyme (horseradish peroxidase) conjugate solution. A solution of tetramethylbenzidine was added and incubated for 20 min, resulting in the development of a blue color. The color development was stopped with the addition of 1N HCl, and the resulting yellow color was measured spectrophotometrically at 450 nm. The concentration of TSH was directly proportional to the color intensity of the test sample.
Diagnosis of subclinical hypothyroidism and exclusion criteria
All dyslipidemic patients and healthy participants were investigated by a physician at Palestinian Medical Relief Society in Gaza.
SCH was diagnosed when serum TSH level >4.7 mIU/L in the presence of normal free thyroid hormone levels (FT4 and FT3).
Patients with overt hypothyroidism or hyperthyroidism, chronic diseases, on hormonal therapy, or pregnant women were excluded from the study.
Ethical consideration
A request letter to execute the study was sent to Palestinian Medical Relief Society administration from Al-Aqsa-University. The study was carried out according to principles of Helsinki Declaration. Participation was voluntary, and participants were given a full explanation about the goals of the study. Confidentiality of all participants was maintained as no names were requested.
Statistical analysis
Data analyses were performed using the Statistical Package for the Social Sciences (SPSS) software (version 17) (SPSS Inc. Chicago, IL, USA). Continuous data were expressed as mean ± standard deviation whereas categorical data as number (%). Comparison of variables between cases and controls was performed with an unpaired t-test for continuous data and Chi-square for categorical data.
Pearson correlation coefficients were calculated for TC, TG, LDL-C, and HDL-C with TSH.
Odd ratios were calculated using Win Episcope (version 2.0, Epidecon, Gobierno De Aragon) to find the association between selected variables (gender, smoking, family history, and remove fat from meat) and dyslipidemia associated with SCH in study participants. P < 0.05 was considered statistically significant.
| Results | |  |
Demographic and clinical data of participants
This case–control study was conducted on forty dyslipidemic patients with a mean age 53 years and another forty age-matched healthy controls with a mean age of 49 years (P = 0.078). The cases characterized by significantly higher body mass index values compared to controls (28.4 ± 2.6 vs. 24.8 ± 1.8 kg/m 2, P = 0.000). In addition, dyslipidemic patients had significant increased systolic blood pressure values in comparison to healthy controls (136.5 ± 23.3 mmHg vs. 121.6 ± 6.6 mmHg, P = 0.000). However, no significant difference in diastolic values was recorded between two groups (P = 0.348).
The patients in case group showed significant lipids alteration when compared to controls. Moreover, dyslipidemic patients manifested significant higher TSH compared to controls (P = 0.049), whereas T3 and T4 levels were not showed significant differences between cases and controls [Table 1].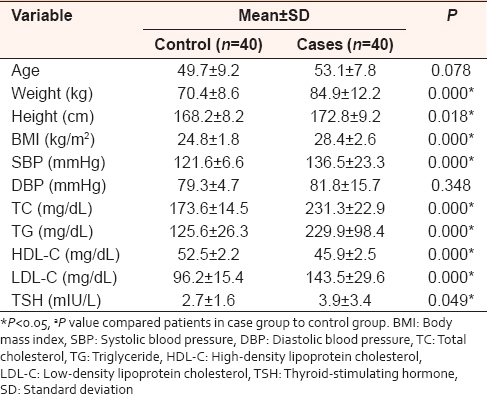 | Table 1: Demographic and clinical characteristics of dyslipidemic patients and matched healthy controls: Data represented as mean±standard deviation
Click here to view |
Relationship between thyroid-stimulating hormone and lipid profile in patients
We performed Pearson correlation analysis between serum concentrations of TSH and lipids profile parameters. Data showed that TSH level has a positive significant correlation with TC (r = 0.445, P= 0.000) and LDL-C (r = 0.415, P = 0.000) but negatively correlated to HDL-C (r = -0.422 and P = 0.000). There is no correlation between TSH and TG [Figure 1]a,[Figure 1]b,[Figure 1]c,[Figure 1]d.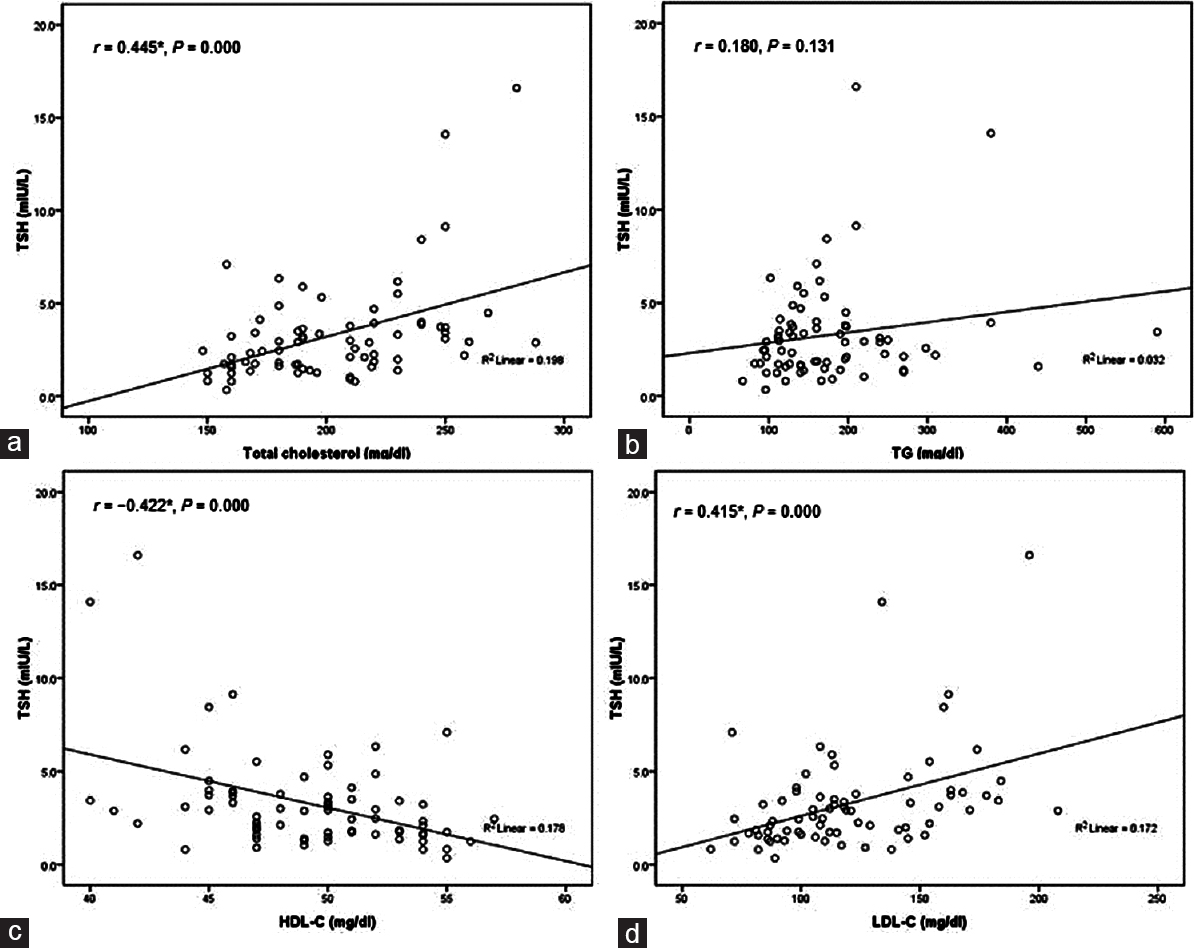 | Figure 1: Thyroid-stimulating hormone (mIU/L) versus serum levels of lipids profile (mg/dL) in dyslipidemic patients and healthy controls (a) the relationship between thyroid-stimulating hormone and total cholesterol (r = 0.445*,P = 0.000); *correlation is significant at the 0.01 level (two tailed); (b) the relationship between thyroid-stimulating hormone and triglycerides (r = 0.180,P = 0.131); correlation is not significant; (c) the relationship between thyroid-stimulating hormone and high-density lipoprotein-C (r = -0.422*,P = 0.000); *correlation is significant at the 0.01 level (two tailed); (d) the relationship between thyroid-stimulating hormone and low-density lipoprotein-cholesterol (r = 0.415*,P = 0.000); *correlation is significant at the 0.01 level (two tailed)
Click here to view |
Factors associated with dyslipidemia and subclinical hypothyroidism
Logistic regression analysis [Table 2] showed that female, smoking, and positive history of dyslipidemia were risk factors for SCH in study participants. | Table 2: Logistic regression model for factors associated with dyslipidemia and subclinical hypothyroidism in study participants (n=80)
Click here to view |
| Discussion | |  |
Thyroid hormones regulate cholesterol and lipoprotein metabolism, whereas thyroid disorders, including overt and SCH, considerably alter lipid profile and promote cardiovascular disease.[4] Although it is estimated that 1%–11% of all patients with dyslipidemia have SCH, the effects of SCH on serum lipid values are less clear and inconsistent.[11]
The present study reported that dyslipidemic patients showed significant higher TSH compared to controls (P = 0.049), whereas T3 and T4 levels were not significantly differ between cases and controls. Our finding confirmed previous studies that reported patients with SCH had significantly lipids alteration pattern.[5],[12],[13] Moreover, a community-based study reported a significant association between SCH and increased serum TC and LDL-C concentrations. Serum TC and LDL-C were significantly increased in patients with SCH compared with euthyroid patient.[14]
The causative mechanisms of the thyroid hormones effects on lipid metabolism consist of an indirect effect through increased expression of Sterol Regulatory Element-Binding Protein-2 (SREBP-2) and a direct effect of T3 on the promoter of the LDL receptor gene.[4]
SREBP-2 is a member of transcription factors family that regulates glucose metabolism, fatty acid synthesis, and cholesterol metabolism.[15] The SREBP-2 gene is regulated by thyroid hormone. It has been reported that the increased SREBP-2 nuclear protein levels in hypothyroid animals result in thyroid hormone-independent activation of LDL receptor gene expression and reversal of the associated hypercholesterolemia.[16] That mean the levels of cellular cholesterol regulate LDL receptor expression through negative feedback inhibition by the SREBP-2 gene.[17] Moreover, the decreased LDL receptor and increased serum cholesterol associated with hypothyroidism are secondary to the thyroid hormone effects on SREBP-2. These results suggest that agents that directly increase SREBP-2 expression can reverse hypercholesterolemia associated with hypothyroidism.[18]
In addition, thyroid hormone to varying degrees increases the activity of the enzymes involved in the metabolism of lipoproteins and reverse cholesterol transport such as hepatic lipase, lipoprotein lipase, cholesteryl ester transfer protein, and lecithin cholesterol acyltransferase.[4] These explanations supported our findings that TSH was strongly associated with higher TC and LDL-C concentrations in dyslipidemic patients as shown in [Figure 1]. Such association between hypothyroidism and increased LDL-C, total serum cholesterol and decrease in HDL-C may enhance the risk for development of atherosclerosis and coronary artery disease although there is no clear evidence to date that hypothyroidism causes clinical heart disease.[4],[19] Even mild elevations of TSH are associated with changes in lipid profile significant enough to raise the cardiovascular risk.
The logistic regression analysis from the present study indicated a role of some risk factors (gender, smoking, and family history of dyslipidemia) in developing of dyslipidemia and hypothyroidism. Dyslipidemic females are 3 times more at risk to develop hypothyroidism compared to males. In support of this finding, Rizos et al.[20] reported that SCH has a higher prevalence among women and older populations.[9] Smokers are 6 times at risk to develop hypothyroidism and dyslipidemia. Smoking and insulin resistance may modify the effects of SCH on serum lipid values.[11]
| Conclusions | |  |
The findings from the present study reflect the association of dyslipidemia and SCH. The routine screening of thyroid profile in dyslipidemic patients will aid in the early diagnosis of thyroid dysfunction and hence the disease management at an early stage.
Acknowledgment
The authors would like to thank the Medical Relieve Society-Gaza, Dr. Gassan El-Astal, Dr. Salem Shubair, and Mr. Abd Alrahman Hamad, for facilitation and helping during samples collection and data analysis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| References | |  |
| 1. | Tabatabaei-Malazy O, Qorbani M, Samavat T, Sharifi F, Larijani B, Fakhrzadeh H. Prevalence of dyslipidemia in Iran: A systematic review and meta-analysis study. Int J Prev Med 2014;5:373-93.  [ PUBMED] |
| 2. | Santos RD, Bensenor IM, Pereira AC, Lotufo PA. Dyslipidemia according to gender and race: The Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). J Clin Lipidol 2016;10:1362-8.  [ PUBMED] |
| 3. | Taheri F, Kazemi T, Bijari B, Namakin K, Zardast M, Chahkandi T. Prevalence of dyslipidemia among elementary school children in Birjand, East of Iran, 2012. J Tehran Heart Cent 2016;11:15-20.  [ PUBMED] |
| 4. | Duntas LH, Brenta G. The effect of thyroid disorders on lipid levels and metabolism. Med Clin North Am 2012;96:269-81.  [ PUBMED] |
| 5. | Pucci E, Chiovato L, Pinchera A. Thyroid and lipid metabolism. Int J Obes Relat Metab Disord 2000;24 Suppl 2:S109-12.  [ PUBMED] |
| 6. | Alamdari S, Amouzegar A, Tohidi M, Gharibzadeh S, Kheirkhah P, Kheirkhah P, et al. Hypothyroidism and lipid levels in a community based study (TTS). Int J Endocrinol Metab 2015;14:e22827.  [ PUBMED] |
| 7. | Cooper DS, Biondi B. Subclinical thyroid disease. Lancet 2012;379:1142-54.  [ PUBMED] |
| 8. | Liberopoulos EN, Elisaf MS. Dyslipidemia in patients with thyroid disorders. Hormones (Athens) 2002;1:218-23.  [ PUBMED] |
| 9. | Ito M, Takamatsu J, Sasaki I, Hiraiwa T, Fukao A, Murakami Y, et al. Disturbed metabolism of remnant lipoproteins in patients with subclinical hypothyroidism. Am J Med 2004;117:696-9.  [ PUBMED] |
| 10. | Aldebasi YH, Mohieldein AH, Almansour YS, Almutairi BL. Dyslipidemia and lipid peroxidation of Saudi type 2 diabetics with proliferative retinopathy. Saudi Med J 2013;34:616-22.  [ PUBMED] |
| 11. | Pearce EN. Update in lipid alterations in subclinical hypothyroidism. J Clin Endocrinol Metab 2012;97:326-33.  [ PUBMED] |
| 12. | Humerah S, Siddiqui A, Khan HF. Pattern of altered lipid profile in patients with subclinical and clinical hypothyroidism and its correlation with body mass index. J Coll Physicians Surg Pak 2016;26:463-6.  [ PUBMED] |
| 13. | Laway BA, War FA, Shah S, Misgar RA, Kumar Kotwal S. Alteration of lipid parameters in patients with subclinical hypothyroidism. Int J Endocrinol Metab 2014;12:e17496.  [ PUBMED] |
| 14. | Walsh JP, Bremner AP, Bulsara MK, O'leary P, Leedman PJ, Feddema P, et al. Thyroid dysfunction and serum lipids: A community-based study. Clin Endocrinol (Oxf) 2005;63:670-5.  |
| 15. | Mullur R, Liu YY, Brent GA. Thyroid hormone regulation of metabolism. Physiol Rev 2014;94:355-82.  [ PUBMED] |
| 16. | Shin DJ, Osborne TF. Thyroid hormone regulation and cholesterol metabolism are connected through Sterol Regulatory Element-Binding Protein-2 (SREBP-2). J Biol Chem 2003;278:34114-8.  [ PUBMED] |
| 17. | Horton JD, Goldstein JL, Brown MS. SREBPs: Activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest 2002;109:1125-31.  [ PUBMED] |
| 18. | Bansal MP, Jaswal S. Hypercholesterolemia induced oxidative stress is reduced in rats with diets enriched with supplement from Dunaliella salina algae. Am J Biomed Sci 2009;1:196-204.  |
| 19. | Suh S, Kim DK. Subclinical hypothyroidism and cardiovascular disease. Endocrinol Metab (Seoul) 2015;30:246-51.  [ PUBMED] |
| 20. | Rizos CV, Elisaf MS, Liberopoulos EN. Effects of thyroid dysfunction on lipid profile. Open Cardiovasc Med J 2011;5:76-84.  [ PUBMED] |
[Figure 1]
[Table 1], [Table 2]
|