|
 
 |
| ORIGINAL ARTICLE |
|
| Year : 2017 | Volume
: 12
| Issue : 2 | Page : 56-60 |
|
Serum bladder tumor antigen levels in subjects with sickle cell anemia: A preliminary report
Patrick O Manafa1, Chide E Okocha2, John C Aneke2, Chijindi Nwakor1, Nancy C Ibeh1, George O Chukwuma1, Ejike K Nwene3
1 Department of Medical Laboratory Science, College of Health Sciences, Nnamdi Azikiwe University, Nigeria
2 Department of Haematology, Nnamdi Azikiwe University Teaching Hospital, Nnewi, Anambra State, Nigeria
3 Department of Clinical Services, Initiative For Good Health (IGH) in Nigeria, Nigeria
| Date of Web Publication | 24-May-2018 |
Correspondence Address:
John C Aneke
Department of Haematology, Nnamdi Azikiwe University Teaching Hospital, Nnewi, PMB 5025, Anambra State
Nigeria
  | Check |
DOI: 10.4103/summ.summ_31_16 
Background: Sickle cell anemia (SCA) has been linked with the occurrence of some tumors, including that of the urological system; the serum bladder tumor antigen has been shown to be a surrogate marker for bladder carcinoma. Objective: The objective of this study was to evaluate serum bladder tumor antigen in SCA subjects in comparison with disease severity and levels in subjects with other hemoglobin phenotypes. Subjects and Methods: A total of 50 subjects were randomly recruited which comprised of 20 homozygous SCA subjects in steady state, 20 heterozygous hemoglobin AS (HbAS), and 10 hemoglobin AA (HbAA) subjects. Five milliliters of venous blood was collected from each participant for hemoglobin type confirmation and estimation of bladder tumor antigen levels, using cellulose acetate hemoglobin electrophoresis and enzyme-linked immunosorbent assay, respectively. Disease severity scoring was based on the earlier report of Okocha et al. Results: The mean serum level of bladder tumor antigen was significantly lower in SCA compared with HbAS and HbAA subjects (23.12 ± 3.75 ng/ml vs. 29.60 ± 3.80 ng/ml and 34.65 ± 4.05 ng/ml, P < 0.001, respectively). Correspondingly, the mean serum bladder tumor antigen levels were significantly lower in HbAS compared with HbAA subjects (P < 0.001). Serum bladder tumor antigen level was not significantly correlated with disease severity in subjects with SCA (r = −0.267, P = 0.318). Conclusion: The low serum levels of bladder tumor antigen in subjects with SCA may indicate a lower risk of bladder carcinoma.
Keywords: Bladder tumor, disease severity, sickle cell anemia
How to cite this article:
Manafa PO, Okocha CE, Aneke JC, Nwakor C, Ibeh NC, Chukwuma GO, Nwene EK. Serum bladder tumor antigen levels in subjects with sickle cell anemia: A preliminary report. Sudan Med Monit 2017;12:56-60 |
How to cite this URL:
Manafa PO, Okocha CE, Aneke JC, Nwakor C, Ibeh NC, Chukwuma GO, Nwene EK. Serum bladder tumor antigen levels in subjects with sickle cell anemia: A preliminary report. Sudan Med Monit [serial online] 2017 [cited 2018 Jul 22];12:56-60. Available from: http://www.sudanmedicalmonitor.org/text.asp?2017/12/2/56/233102 |
| Introduction | |  |
Sickle cell disease (SCD) is a genetic disorder of hemoglobin, characterized by chronic anemia, acute painful episodes, organ infarction with chronic organ damage, and significant reduction in life expectancy.[1],[2] It is the most common genetic disorder of hemoglobin in sub-Saharan Africa, where up to 200,000 babies are born with the disease annually.[3],[4],[5]
A point mutation on the 6th codon of the beta-globin gene located on the short arm of chromosome 11 is responsible for the development of the disease. The mutation produces a defective beta-globin chain, which under low oxygen tension polymerizes into long fibers that eventually lead to abnormally deformed (sickled) red cells. The sickled red cells become sticky, adhere to the endothelium, and clump together plugging microvessels and equally causing damage to larger blood vessels.[6] In addition to vasculopathy, sickle red cells have markedly reduced survival in circulation, resulting in a chronic hemolytic anemia state. The interaction of sickled red cells with the vascular endothelium leads to episodic microvascular occlusion, ischemia with reperfusion, vascular and inflammatory stress, and ultimately a myriad of potentially life-threatening clinical manifestations.[6],[7]
A number of recent reports have shown the utility of a monoclonal antibody-based (mAb-based) assay (BTA-TRAK) for the detection of bladder cancer.[8] The mAb used in the assay targets the bladder tumor antigen (also known as soluble human complement factor H [hCFH] protein) which had entered the urine of patients with bladder cancer from the plasma.[9]
Plasma hCFH is a 150-kDa protein translated from a 4.4 kb mRNA; it is composed of 20 domains called short consensus repeats.[8] The protein is an efficient regulator of the activity of the alternative pathway C3 and C5 convertases in plasma and equally on the surfaces of host cells.[9] It does this by preventing factor B binding to C3b, thereby promoting dissociation of the C3bBb enzyme complex (decay accelerating activity) and equally by acting as a cofactor for factor I-mediated inactivation of C3b. Through its activity against the alternate pathway, the hCFH protein promotes carcinogenesis by binding to tumor cells in the immediate vicinity of the tumor microenvironment and protects these cells from complement-mediated cell lysis, a critical component of normal body tumor surveillance mechanism.[10],[11]
Information on the prevalence of malignancies in patients with SCD is sparse; a recent multicenter study reported the prevalence rate of renal medullary carcinoma in Nigerian SCD subjects to be 5.6/100,000.[12] Similarly, a single institution study done among SCD subjects in the United States of America reported a prevalence rate of malignancy of 1.74/1000 patient-years.[13] There has been no study that specifically screened for bladder carcinoma in our population of SCD patients, using surrogate markers of the disease.
In view of some reports of malignancies in subjects with SCD, this pilot study was, therefore, designed to bridge this knowledge gap, by screening for the bladder tumor antigen (hCFH) in subjects with different hemoglobin phenotypes and comparing serum levels with disease severity (in subjects with sickle cell anemia [SCA]). We hope that findings from this pilot may highlight the need for a larger multicenter study on this subject in the future.
| Subjects and Methods | |  |
Study area
This was the Nnamdi Azikiwe University Teaching Hospital, (NAUTH), a government owed, tertiary healthcare facility in Nnewi, Anambra State, Nigeria.
Research design
A total of 50 subjects were randomly recruited from our weekly hematology outpatient clinic and from among consenting members of the hospital community. These included 20 confirmed homozygous SCA patients in steady state, 20 heterozygous hemoglobin AS (HbAS) subjects, and 10 hemoglobin AA (HbAA) individuals who served as the control group. The selection criteria for SCA subjects in the steady-state group were based on the absence of any form of crisis for at least 3 weeks and no blood transfusion, 4 weeks before recruitment. The information utilized for severity scoring was obtained from questionnaires administered to the subjects, as adapted from the earlier report of Okocha et al.[14] The parameters used for calculating severity score included the hemoglobin concentration, total white cell count, number of vaso-occlusive crisis, lifetime blood transfusion rate, and sickle-related complications. Subjects with score ≤3 were classified as mild, those with score of >3–≤7 were classified as moderate, while those with score >7 were classified as severe disease.
Ethical consideration
The ethical approval for this research was sought from the NAUTH Ethics Committee, and each subject gave written informed consent at the point of recruitment.
Sample collection and processing
About 5 ml of venous blood was collected from each participant following the standard protocol for venesection; 2 ml was dispensed into ethylene diamine tetra acetic acid (EDTA) specimen container for full blood count (FBC) and hemoglobin phenotype determination, while the remaining 3 ml was collected into plain container and spurn at 3000 rpm for 5 min. Serum was extracted and used for the determination of bladder tumor antigen levels. FBC and hemoglobin phenotype of each participant were done using automated hematology analyzer (Mythic 22, Switzerland ®) and cellulose acetate paper electrophoresis (Helena Biosciences, UK ®), respectively. Estimation of bladder tumor antigen levels was based on immunometric double-antibody “sandwich” technique, using factor H human enzyme-linked immunosorbent assay test kits procured from Abcam diagnostics ® Inc., USA. The test protocol involved the addition of standards and samples on antibody-coated plates followed by incubation. Horseradish peroxide-labeled factor H monoclonal antibody was subsequently added after the plates were properly rinsed. A “sandwich” was formed by the two antibodies, followed by the addition of a chromogenic substrate and stop solution. The reaction mix was gently mixed for 30 s and read at 450 nm using a microplate reader.
Statistical analysis
Data analysis was done using the Statistical Package for the Social Sciences, version 20 computer software (SPSS Inc., Chicago, IL, USA) and results of serum bladder tumor antigen were presented as means ± standard deviation. Comparison of means of bladder tumor antigen among the various hemoglobin phenotypes was done using the Student's t-test and analysis of variance as appropriate, while the relationship between antigen level and objective scores of disease severity (in SCA only) was determined by the Pearson's linear regression for bivariate correlation; P < 0.05 was accepted as statistically significant.
| Results | |  |
The means of serum bladder tumor antigen were significantly lower in subjects with SCA compared with those with HbAS hemoglobin type [23.86 ± 8.47 ng/ml vs. 29.60 ± 3.80 ng/ml respectively, [Table 1]. Correspondingly, serum bladder tumor antigen level was significantly higher in subjects with HbAA compared with those with SCA and HbAS [34.65 ± 4.05 ng/ml vs. 23.86 ± 8.47 ng/ml and 29.60 ± 3.80 ng/ml, respectively, [Table 1].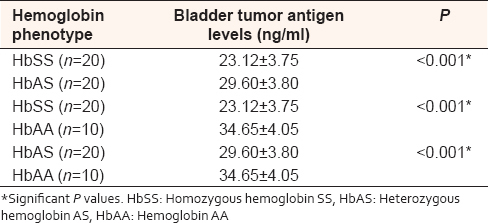 | Table 1: Comparison of serum bladder tumor antigen in subjects with different hemoglobin phenotypes
Click here to view |
Serum bladder tumor antigen levels were negatively correlated with objective score of disease severity in subjects with homozygous hemoglobin SS (r = −0.267); this was, however, not statistically significant (P = 0.318) [Figure 1].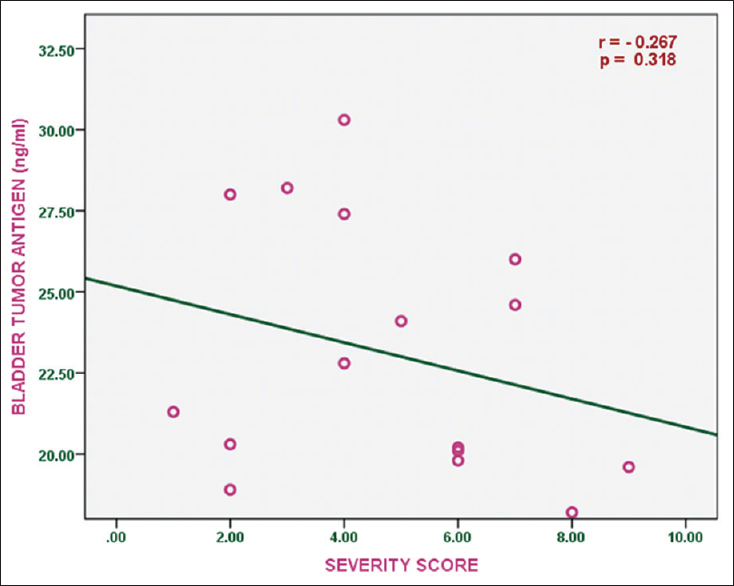 | Figure 1: Correlation of serum bladder antigen levels with disease severity score in subjects with sickle cell anemia
Click here to view |
There was no statistically significant difference in the serum levels of bladder tumor antigen in SCA subjects with mild, moderate, and severe disease [23.34 ± 4.43 ng/ml, 22.03 ± 1.61 ng/ml, and 18.90 ± 0.99 ng/ml, respectively, P = 0.24, [Figure 2].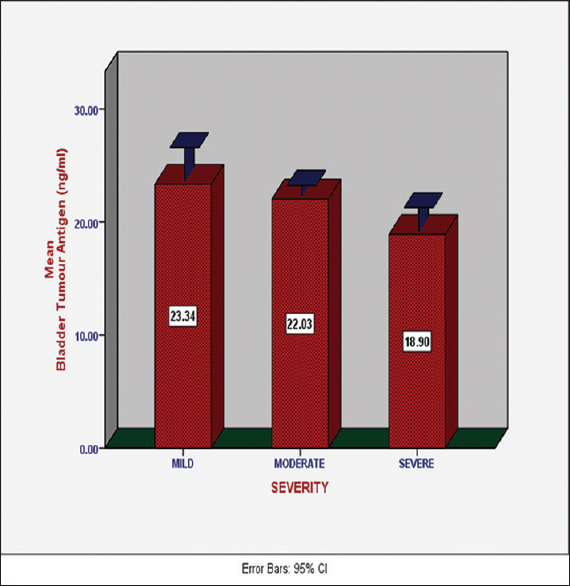 | Figure 2: Serum levels of bladder tumor antigen in sickle cell anemia subjects with different degrees of disease severity
Click here to view |
| Discussion | |  |
Historically, the development of malignancy in persons living with SCD has been documented over the past 50–60 years.[15],[16],[17],[18] These reports were at best scattered and neither provided complete data on the types of cancer nor attempted to define the epidemiology of specific cancers that affect subjects with SCD. On the basis of a single institution study, the cancer incidence among patients with SCD has been reported to be 1.74 cases/1000 patient-years by Dawkins et al., at the Howard University Hospital, USA.[13] Similarly, Schultz and Wareidentified 52 cases of cancer among 16,613 patients with SCD followed at 52 institutions in the United States of America and reported a preponderance of carcinomas among adult study subjects.[19] While the above studies emphasized the general prevalence of all cancers in SCD subjects, the multicenter study of Anazoeze et al. reported the prevalence of 5.6/100,000 for renal medullary carcinoma among adult Nigerian SCD population; this was, however, not higher than the general population prevalence.[12] Some factors have been hypothesized to potentially contribute to the occurrence of cancer in SCD subjects, ranging from transfusion transmissible viruses (which may be acquired through recurrent blood transfusions) to the chronic inflammation of SCD and other comorbid variables such as smoking and use of hydroxyurea.[19] To date, there is insufficient comprehensive compilation of malignancy in patients with SCD, using data from multiple institutions with a large patient-year denominator to establish definitive associations.
In this study, serum bladder tumor antigen (which is equally a component of the alternate complement pathway protein) was significantly lower in SCA compared with HbAS and HbAA subjects [Table 1]. Other studies had emphasized lower serum levels of alternate complement pathway proteins in SCA subjects.[20] The lower serum levels of these proteins are thought to significantly increase the infectious risk in subjects with SCA and are believed to result from excess consumption due to the activation of the alternate pathway.[20] The complement system is a central part of the immune system that has developed as a first line of defense against nonself cells, including tumor cells.[21] It can be activated by one of the three pathways, the classical, lectin, and alternative pathways.[22] Low levels of serum complement proteins (especially those of the alternate pathway) have equally been documented in some malignant conditions, and this significantly increases disease-related morbidity due to high infection risk.[23],[24],[25] Infarct, low complement levels (with suboptimal complement activation) have been shown to allow cancer cells to escape complement-mediated elimination and equally hamper the clinical efficacy of monoclonal antibody-based cancer immunotherapies.[21] In view of the established role of bladder tumor antigen in creating enabling environment for tumor growth (through inhibition of complement-mediated tumor surveillance) vis-a-vis the low serum levels observed in our subjects, we hypothesize that bladder carcinoma may not be common in our SCA population. Although our finding is in keeping with the report of Anazoeze et al., it contrasts other studies which appear to suggest that SCD subjects have increased tendency to develop malignant conditions.[12],[15],[16],[17],[18],[19],[26]
Interestingly, serum levels of bladder tumor antigen were not significantly correlated with disease severity in subjects with SCA (r = −0.267, P = 0.318). We did not find this surprising as an intact complement system (devoid of the inhibitory effects of bladder tumor antigen) could significantly reduce infection risk and engender a less severe disease phenotype. Infection (including malaria, in endemic parts of the world) is a significant contributor to morbidity in SCA subjects.[27],[28]
| Conclusion | |  |
In contrast to a number of earlier reports which suggested higher risk of malignancy in subjects with SCA, the low serum level bladder tumor antigen (a surrogate marker for bladder carcinoma which is believed to encourage tumor growth through inhibition of complement-mediated tumor surveillance) observed in this study could suggest that bladder carcinoma may not be common in our population. We recommend larger multicenter studies to confirm this finding.
Strength of the study
This is the first study that evaluated the risk of bladder carcinoma among African steady-state SCA subjects, in comparison with disease severity.
Limitation of the study
Due to the small sample size, these results are preliminary and will need to be confirmed by larger (possibly multicenter) studies.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| References | |  |
| 1. | Chakravorty S, Williams TN. Sickle cell disease: A neglected chronic disease of increasing global health importance. Arch Dis Child 2015;100:48-53.  [ PUBMED] |
| 2. | Aneke JC, Adegoke AO, Oyekunle AA, Osho PO, Sanusi AA, Okocha EC, et al. Degrees of kidney disease in Nigerian adults with sickle-cell disease. Med Princ Pract 2014;23:271-4.  [ PUBMED] |
| 3. | Okocha C, Onubogu CU, Aneke J, Onah C, Ajuba I, Ibeh N et al. Prevalence of sickle cell gene among apparently healthy under-two South-East Nigerian children: What is the role of parental premarital counseling and socio-demographic characteristics? Nig J Med 2016:25;176-81.  |
| 4. | Diallo D, Tchernia G. Sickle cell disease in Africa. Curr Opin Hematol 2002;9:111-6.  [ PUBMED] |
| 5. | Aneke JC, Okocha CE. Sickle cell disease genetic counseling and testing: A review. Arch Med Health Sci 2016;4:50-7.  [Full text] [Full text] |
| 6. | Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet 2010;376:2018-31.  [ PUBMED] |
| 7. | Aneke JC, Huntley N, Porter J, Eleftheriou P. Effect of automated red cell exchanges on oxygen saturation on-air, blood parameters and length of hospitalization in sickle cell disease patients with acute chest syndrome. Niger Med J 2016;57:190-3.  [ PUBMED] [Full text] |
| 8. | Raitanen MP, Marttila T, Nurmi M, Ala-Opas M, Nieminen P, Aine R, et al. Human complement factor H related protein test for monitoring bladder cancer. J Urol 2001;165:374-7.  [ PUBMED] |
| 9. | Kinders R, Jones T, Root R, Bruce C, Murchison H, Corey M, et al. Complement factor H or a related protein is a marker for transitional cell cancer of the bladder. Clin Cancer Res 1998;4:2511-20.  [ PUBMED] |
| 10. | Guo A, Wang X, Gao L, Shi J, Sun C, Wan Z, et al. Bladder tumour antigen (BTA stat) test compared to the urine cytology in the diagnosis of bladder cancer: A meta-analysis. Can Urol Assoc J 2014;8:E347-52.  |
| 11. | Junnikkala S, Jokiranta TS, Friese MA, Jarva H, Zipfel PF, Meri S, et al. Exceptional resistance of human H2 glioblastoma cells to complement-mediated killing by expression and utilization of factor H and factor H-like protein 1. J Immunol 2000;164:6075-81.  |
| 12. | Anazoeze M, Najibah G, Garba U, Shehu A, Florence F, Abdulaziz H, et al. Is renal medullary carcinoma the seventh nephropathy in sickle cell disease? A multi-center Nigerian survey. Afr Health Sci 2016;16:490-6.  [ PUBMED] |
| 13. | Dawkins FW, Kim KS, Squires RS, Chisholm R, Kark JA, Perlin E, et al. Cancer incidence rate and mortality rate in sickle cell disease patients at howard university hospital: 1986-1995. Am J Hematol 1997;55:188-92.  [ PUBMED] |
| 14. | Okocha E, Onwubuya E, Osuji C, Ahaneku G, Okonkwo U, Ibeh N, et al. Disease severity scores and haemogram parameters in Nigerian sickle cell disease patients. J Blood Disord Transfus 2015;6:324. Available from: http://www.omicsonline.org. [Last assessed on 2016 Nov 04].  |
| 15. | Baron BW, Mick R, Baron JM. Hematuria in sickle cell anemia – Not always benign: Evidence for excess frequency of sickle cell anemia in African Americans with renal cell carcinoma. Acta Haematol 1994;92:119-22.  [ PUBMED] |
| 16. | Anderson IS, Yeung KY, Hillman D, Lessin LS. Multiple myeloma in a patient with sickel cell anemia. Interacting effects on blood viscosity. Am J Med 1975;59:568-74.  [ PUBMED] |
| 17. | Stricker RB, Linker CA, Crowley TJ, Embury SH. Hematologic malignancy in sickle cell disease: Report of four cases and review of the literature. Am J Hematol 1986;21:223-30.  [ PUBMED] |
| 18. | Goldin AG, Kelty KC, Beard MF. Sickle cell anemia terminating in acute myeloblastic leukemia. Ann Intern Med 1953;39:920-8.  [ PUBMED] |
| 19. | Schultz WH, Ware RE. Malignancy in patients with sickle cell disease. Am J Hematol 2003;74:249-53.  [ PUBMED] |
| 20. | Dieye TN, Ndiaye O, Ndiaye AB, Thiam D, Fall-Seck K, Diop S, et al. Complement and serum immunoglobulins in homozygous and heterozygous sickle cell anemia in Senegal. Dakar Med 1999;44:175-9.  [ PUBMED] |
| 21. | Pio R, Corrales L, Lambris JD. The role of complement in tumor growth. Adv Exp Med Biol 2014;772:229-62.  [ PUBMED] |
| 22. | Adamiak M, Abdelbaset-Ismail A, Suszynska M, Abdel-Latif A, Ratajczak J, Ratajczak MZ, et al. Novel evidence that the mannan-binding lectin pathway of complement activation plays a pivotal role in triggering mobilization of hematopoietic stem/progenitor cells by activation of both the complement and coagulation cascades. Leukemia 2017;31:262-5.  |
| 23. | Heath ME, Cheson BD. Defective complement activity in chronic lymphocytic leukemia. Am J Hematol 1985;19:63-73.  [ PUBMED] |
| 24. | Zurlo JJ, Schechter GP, Fries LF. Complement abnormalities in multiple myeloma. Am J Med 1989;87:411-20.  [ PUBMED] |
| 25. | Cheson BD, Walker HS, Heath ME, Gobel RJ, Janatova J. Defective binding of the third component of complement (C3) to streptococcus pneumoniae in multiple myeloma. Blood 1984;63:949-57.  [ PUBMED] |
| 26. | Davis CJ Jr., Mostofi FK, Sesterhenn IA. Renal medullary carcinoma. The seventh sickle cell nephropathy. Am J Surg Pathol 1995;19:1-1.  |
| 27. | Tewari S, Brousse V, Piel FB, Menzel S, Rees DC. Environmental determinants of severity in sickle cell disease. Haematologica 2015;100:1108-16.  [ PUBMED] |
| 28. | Ramakrishnan M, Moïsi JC, Klugman KP, Iglesias JM, Grant LR, Mpoudi-Etame M, et al. Increased risk of invasive bacterial infections in African people with sickle-cell disease: A systematic review and meta-analysis. Lancet Infect Dis 2010;10:329-37.  |
[Figure 1], [Figure 2]
[Table 1]
|