Matters of the Heart: The Case of TNFα-Targeting Drugs
Table 1
Anti-TNFα Drugs and Approved Indications
| Drug | Class | Structure | Approved Indications | |
|---|---|---|---|---|
| Infliximab (Remicade®) | MoAb | Chimeric (human-murine) recombinant anti-TNFα IgG1 |
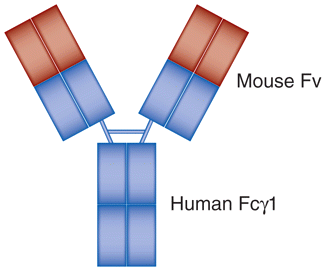 |
Rheumatoid arthritis, Crohn’s disease, ulcerative colitis, ankylosing spondylitis, psoriasis, psoriatic arthritis, Bechet disease |
| Adalimumab (Humira®) | MoAb | Fully human recombinant anti-TNFα IgG1 |
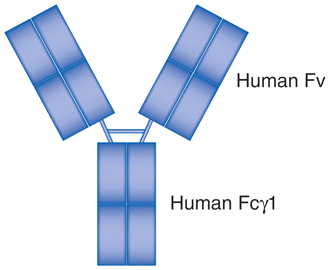 |
Rheumatoid arthritis, juvenile idiopathic arthritis, Crohn’s disease, ulcerative colitis, ankylosing spondylitis, psoriasis, psoriatic arthritis. |
| Golimumab (Simponi®) | MoAb | Fully human recombinant anti-TNFα IgG1 |
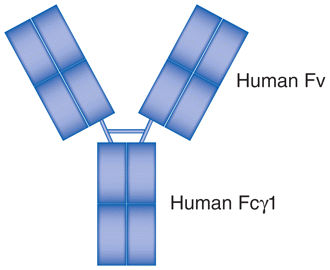 |
Rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis. |
| Certolizumab-pegol (Cimzia®) | Fab’ fragment | Humanized PEGylated Fab’ fragment of anti-TNFα IgG1 |
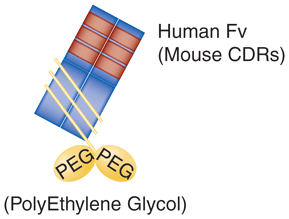 |
Rheumatoid arthritis, Crohn’s disease |
| Etanercept (Enbrel®) | Fusion protein | Fully human TNFR2 linked to IgG1 Fc |
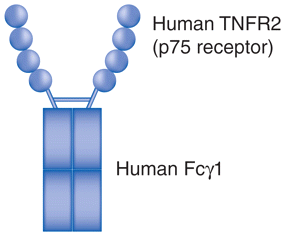 |
Rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriasis, psoriatic arthritis. |
-
MoAb, monoclonal antibody; Fab, fragment, antigen binding; TNFα, tumor necrosis factor α; TNFR2, TNFα receptor 2.



