
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
Vasomotion: Mechanisms and Physiological Importance
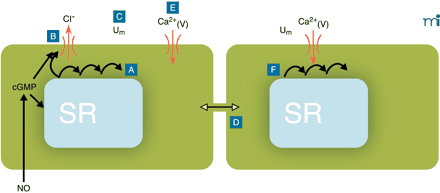
Conceptual model for the generation of vasomotion in rat mesenteric small arteries. The basic oscillator is the sarcoplasmic reticulum (SR), which intermittently releases calcium leading to regenerative waves of calcium traversing the cell (A). In the presence of cyclic GMP (cGMP), this calcium also is able to activate a chloride channel in the plasma membrane (B). If this current is activated in a sufficient number of cells at the same time, cells will depolarize (C). Also cells that are not contracting at that moment will depolarize, because all cells are electrically coupled (D). Depolarization will cause calcium influx through L-type calcium channels (E), which not only triggers contraction but also calcium release in quiescent cells (F), thus resetting their intracellular oscillators. In this way, the likelihood for a sufficient number of cells becoming active together in the next cycle is very high, and the cells in effect become phase-locked. cGMP promotes vasomotion by its permissive action on the ion channel, most likely via protein kinase G–mediated phosphorylation of the channel, but inhibits vasomotion by actions on the SR by influencing intracellular calcium availability, uptake, and/or release. Differences in this balance may explain the variable influence of endothelium in vasomotion. Ca2+(V), voltage-gated (L-type) calcium channel; Um, membrane potential.


