
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
Sodium-Dependent Neurotransmitter TRANSPORTERS: OLIGOMERIZATION as a Determinant of Transporter Function and Trafficking
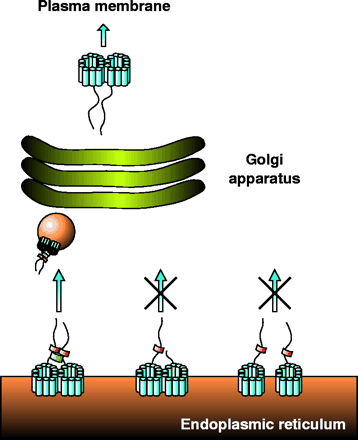
Trafficking of NSS proteins along the secretory pathway. This scheme shows the proposed model of NSS trafficking based on our results obtained with GAT-1. GAT-1 is synthesized in the ER and forms homo-oligomers. For simplicity, GAT-1 is shown as a dimer, although our recent data on DAT and SERT suggest NSS tetramers. The organization of the twelve membrane-spanning segments (blue) is unknown, and therefore the arrangement shown is merely for symbolic purposes. The ER export signal within the C terminus (shown as linear tail) of the transporter recruits the soluble Sec23/24 complex (red). Formation of a stable oligomer is proposed to bring the export signals with their Sec23/24 complexes into close proximity. This proximity in turn allows for the Sec13/31 complex (green) to bridge the Sec23/24 complexes, leading to the formation of a stable COPII coat and subsequent budding and transport of a COPII vesicle (orange sphere) to the Golgi apparatus. After modification in the Golgi apparatus, the NSS oligomers are targeted to the plasma membrane. The middle part of the figure illustrates the case in which a protomer in the oligomer lacks the export signal, so that no Sec23/24 complex is recruited and the assembly of a stable COPII coat is blocked; transporters are thus retained in the ER. The right part of the scheme illustrates the case in which either oligomer formation is inhibited or the interactions among protomers are weakened. In this case, each NSS protomer can still recruit Sec23/24 complexes, but subunits are too far away from each other for the Sec13/31 complex to bridge them efficiently. Under these conditions, formation of a stable COPII coat is not efficient and transporters are retained in the ER.


