
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
LIPID Arrays: New Tools in the Understanding of Membrane Dynamics and Lipid Signaling
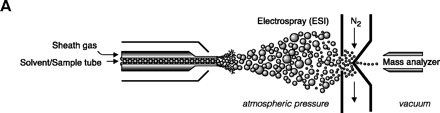
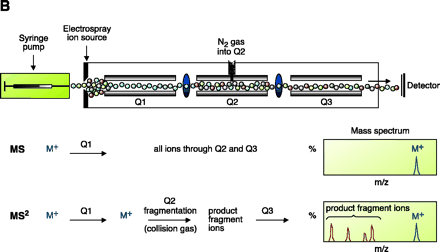
Electrospray ionization and triple quadrupole mass spectrometer. ESI-MS is unsurpassed for complete analysis of phospholipid mixtures. It has been successfully used for quantitative measurements of phospholipids from biological samples (40–43). The “soft” electrospray ionization causes little or no fragmentation and hence virtually all phospholipid species can be detected as molecular ion species (denoted as M*), when analyzed in a single-stage mode using either Q1 or Q3 quadrupoles operating as mass analyzers. The choice of polarity is determined by the possible charge state of the phospholipid class in solution. All zwitterionic phospholipids (PC, LPC, SM, and PE) can ionize in both positive and negative ion modes. With the exception of PE, they are more efficiently analyzed in positive mode. Because of their negative charge at neutral pH, anionic phospholipids (PI, PA, PG, and PS) produce primarily [M-H]- (deprotonated molecule retaining a negative charge) as the molecular ion peak and are easily detected in negative ion mode. Thus, two sets of mass spectra are obtained for each sample—one in positive and one in negative ionization mode, with all detected phospholipid species presented as peaks at their specific m/z value. The identification and structural information about a particular peak is acquired by tandem MS (44, 45). The relevant ion is subjected to collision-induced dissociation (CID) by interaction with a collision gas. A fragmentation spectrum (product, or “daughter” spectrum) is generated as a result, revealing structural information (acyl chain or head group chemistry, depending on the ionization mode). On that basis, “fragmentation libraries” can be created for the phospholipid species composition of any cell type (38).


